Question: pls help!!!! The given first order reaction, 2A products has the following rate low: r=0.622s1[A] What is the half-life of the reaction when the concentration
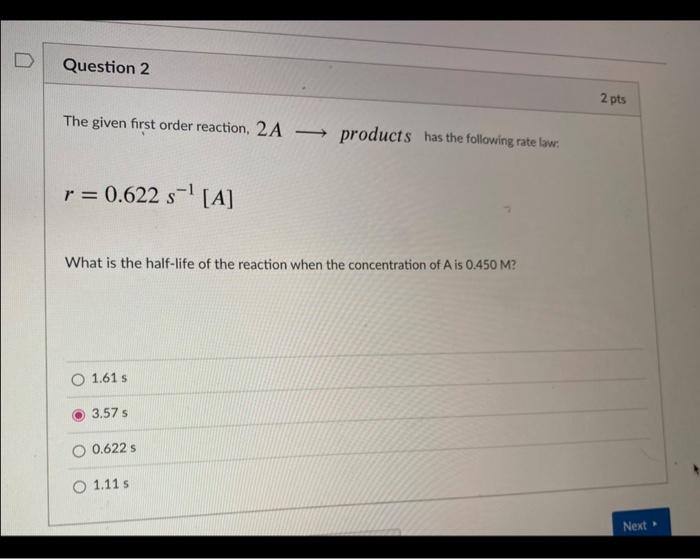
![following rate low: r=0.622s1[A] What is the half-life of the reaction when](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90a9c4df08_21166f90a9bed147.jpg)
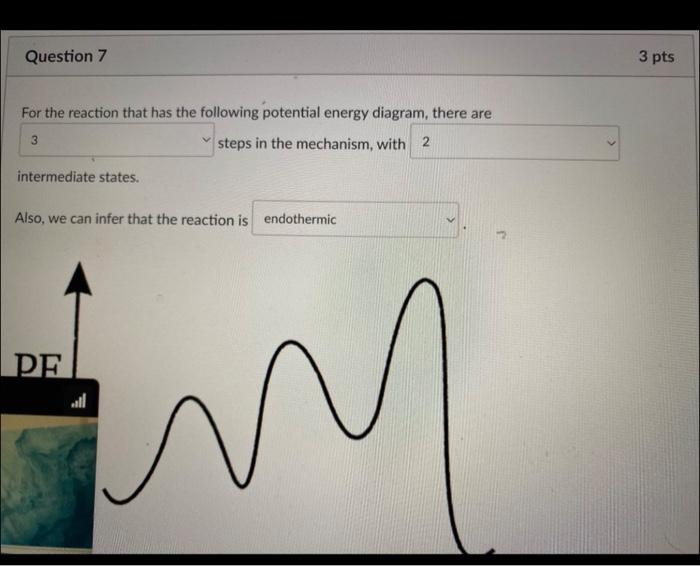
The given first order reaction, 2A products has the following rate low: r=0.622s1[A] What is the half-life of the reaction when the concentration of A is 0.450M ? 1.61s 3.57s 0.622s 1.11s The presence of a catalyst provides a mechanism with a activation energy and thus causes a reaction rate. higher, higher lower, steady higher, lower lower, higher lower, lower For the reaction that has the following potential energy diagram, there are steps in the mechanism, with intermediate states. Also, we can infer that the reaction is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


