Question: pls help with both, important !!:( Dinituogen pentoxde decomposes in the qgas phase to form nitrogen dioxide-and oxygen gap. The feactibe is first arder in
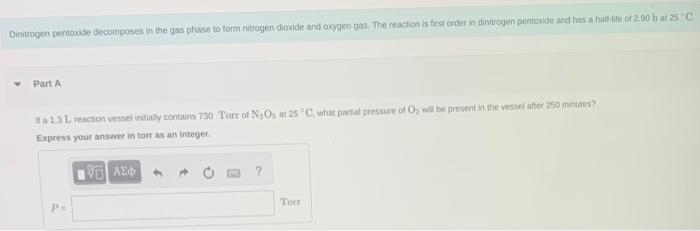

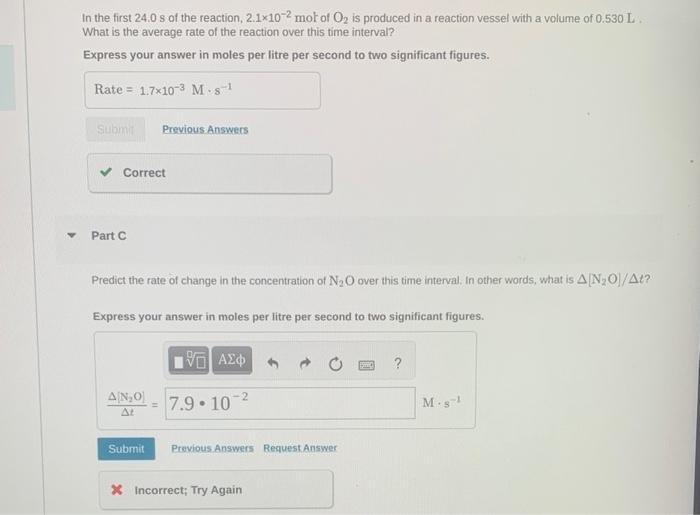
Dinituogen pentoxde decomposes in the qgas phase to form nitrogen dioxide-and oxygen gap. The feactibe is first arder in dinitrogen pentaxide and has a hall-file of 2.90 b at 2. . Part A it a 13L reaction vessel intialy contains 730 Tort of N2O5 ar 255C, what partial pressure of O2 will be present in the vessel ahter 250 minutes? Express your answer in torr as an integer. Consider the following reaction: 2N2O(g)2N2(g)+O2(g) In the first 24.0s of the reaction, 2.1102mol of O2 is produced in a reaction vessel with a volume of 0.530L. What is the average rate of the reaction over this time interval? Express your answer in moles per litre per second to two significant figures. Part C Predict the rate of change in the concentration of N2O over this time interval, In other words, what is [N2O]/t ? Express your answer in moles per litre per second to two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


