Question: pls help witn both A reaction is performed to study the reaction of mercury(II) chloride with oxalate ion: 2HgCl2+C2O422Cl+Hg2Cl4+2CO2 The following reaction rate data was
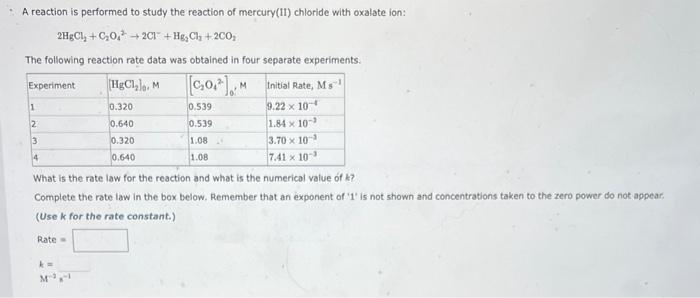

A reaction is performed to study the reaction of mercury(II) chloride with oxalate ion: 2HgCl2+C2O422Cl+Hg2Cl4+2CO2 The following reaction rate data was obtained in four separate experiments. What is the rate law for the reaction and what is the numerical value of k ? Complete the rate law in the box below. Remember that an exponent of ' 1 ' is not shown and concentrations taken to the zero power do not appear. (Use k for the rate constant.) Rate = k= M3n1 The rearrangement of cyclopropane to propene at 500C is first order in (CH2)3. (CH2)3(g)CH3CH=CH2(g) During one experiment it was found that the (CH2)3 concentration dropped from 0.223M at the beginning of the experiment to 7.56102M in 1.80103 s. Wha is the value of the rate constant for the reaction at this temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


