Question: pls help You have to prepare a pH5.04 buffer, and you have the following 0.10M solutions available: HCOOH,HCOONa,CH3COOH, CH3COONa,HCN, and NaCN. How many millitters of
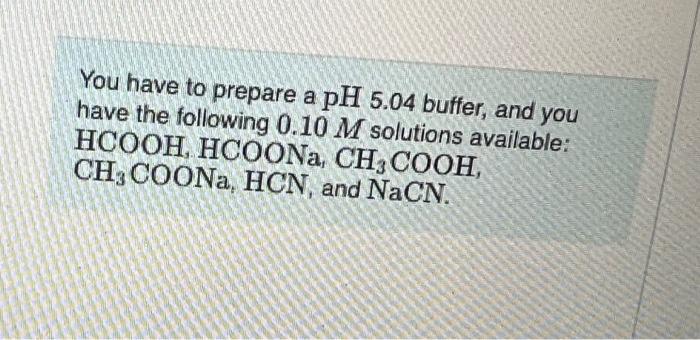
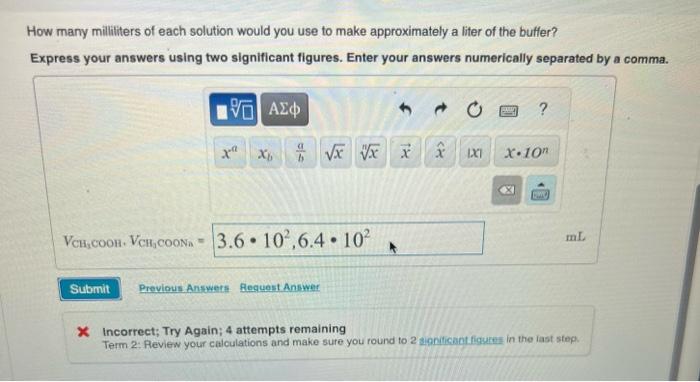
You have to prepare a pH5.04 buffer, and you have the following 0.10M solutions available: HCOOH,HCOONa,CH3COOH, CH3COONa,HCN, and NaCN. How many millitters of each solution would you use to make approximately a liter of the butfer? Express your answers using two significant figures. Enter your answers numerically separated by a comma. VCH3COOHCOHVCH2COONin x Incorrect; Try Again; 4 attempts remaining Term 2: Peview your calculations and make sure you round to: In the last stop
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


