Question: pls i need an answer in 2 hours The irreversible exothermic reaction takes place in a continuous stirred tank reactor. The heat released by the
pls i need an answer in 2 hours
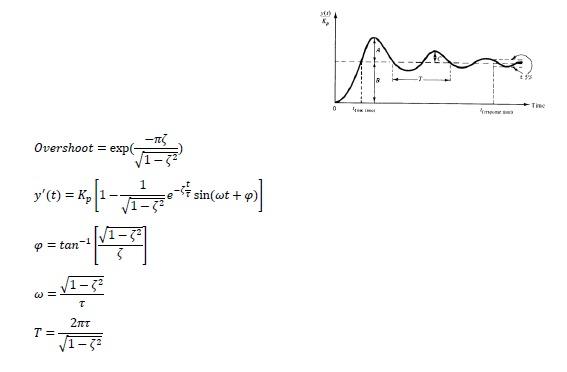
The irreversible exothermic reaction takes place in a continuous stirred tank reactor. The heat released by the reaction is removed using cooling water. A proportional controller is used to adjust coolant flow rate so as to keep the reactor temperature at desired value. The controller has been designed so that the controlled reactor exhibits typical underdamped second-order temperature response characteristics when it is disturbed by feed flow rate. a. The mass feed flow rate to CSTR varies suddenly from 0.3 to 0.4kg/s, and the temperature of the reaction mixture, initially at 90C, increases to 92C. What is the gain of the transfer function that relates changes in reactor temperature to changes in feed flow rate? (Remember transfer function of second order process and use final value theorem) b. The operator notes that the resulting response is slightly oscillatory with a value of 0.25 (A/B in Figure) occurring at times 1000 and 3060s after the change is initiated. Find damping factor and natural period of oscillation. Overshoot=exp(12)y(t)=Kp[1121etsin(t+)]=tan1[12]=12T=122
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


