Question: pls show your work and explain. For a given reaction, H=12.2kJ/mol,S=3.2J/molK, Calculate G at T=393.6K in Joules For a given reaction, G=11.2kJ/mol,S=12.5J/mol at T=419.7K Determine
pls show your work and explain.
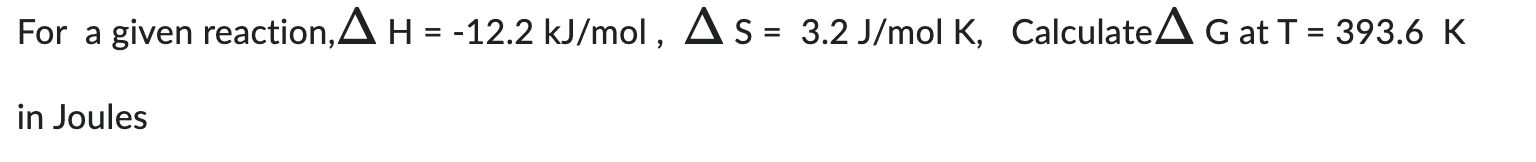

For a given reaction, H=12.2kJ/mol,S=3.2J/molK, Calculate G at T=393.6K in Joules For a given reaction, G=11.2kJ/mol,S=12.5J/mol at T=419.7K Determine H in Joules/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


