Question: plz help my data first page Lab Section_514 Data mass of empty glass-stoppered weighing bottle 35.12669 mass of glass-stoppered weighing bottle filled with deionized water
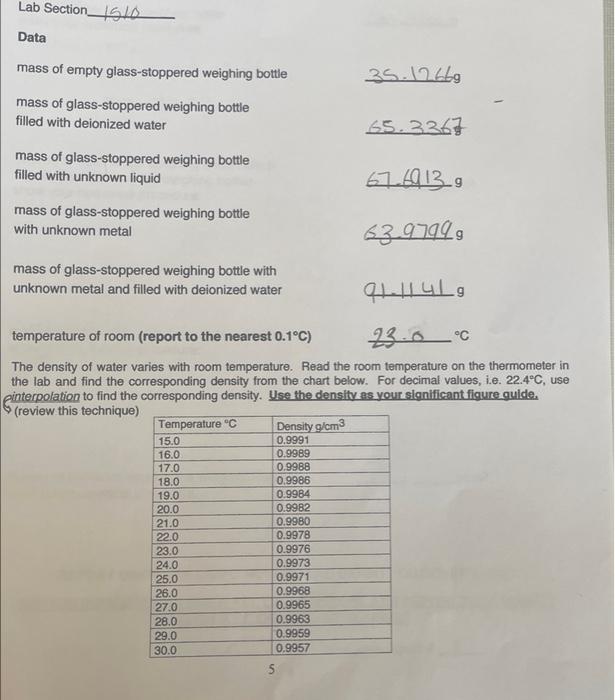



Lab Section_514 Data mass of empty glass-stoppered weighing bottle 35.12669 mass of glass-stoppered weighing bottle filled with deionized water SS. 3267 mass of glass-stoppered weighing bottle filled with unknown liquid 67.4913.9 mass of glass-stoppered weighing bottle with unknown metal 53.97999 mass of glass-stoppered weighing bottle with unknown metal and filled with deionized water 149 temperature of room (report to the nearest 0.1C) 23.0C The density of water varies with room temperature. Read the room temperature on the thermometer in the lab and find the corresponding density from the chart below. For decimal values, i.e. 22.4C, use einterpolation to find the corresponding density. Use the density as your significant figure guide. (review this technique) Temperature "C Density g/cm3 0.9991 15.0 16.0 17.0 18.0 19.0 20.0 21.0 22.0 23.0 24.0 25.0 26.0 27.0 28.0 29.0 30.0 0.9989 0.9988 0.9986 0.9984 0.9982 0.9980 0.9978 0.9976 0.9973 0.9971 0.9968 0.9965 0.9963 0.9959 0.9957 5 Experimental Calculations: PLEASE BE NEAT AND WATCH SIGNIFICANT FIGURES. ****ALL CALCULATIONS MUST BE SHOWN **** Please do NOT use scientific notation or "e" values. Report values as decimals. mass of water in the weighing bottle show your numerical procedure: mw-me 65.3361-35-1266 30-2101g 2 volume of water in the weighing bottle (which equals the volume of the weighing bottle) show your numerical procedure: cm mass of unknown liquid in the weighing bottle show your numerical procedure: g density of unknown liquid in the weighing bottle show your numerical procedure: g/cm3 REPORT UNKNOWN LIQUID NUMBER AND DENSITY ON COVER SHEET. DID YOU REMEMBER TO SHOW ALL CALCULATIONS AND REPORT THE CORRECT NUMBER OF SIGNIFICANT FIGURES? unknown liquid #_14 7 Experimental Calculations Continued mass of unknown metal in the weighing bottle show your numerical procedure: 9 mass of water in the weighing bottle which also contains the unknown metal show your numerical procedure: volume of water in the weighing bottle which also contains the unknown metal show your numerical procedure: cm3 cm3 volume of unknown metal in the weighing bottle Hint: compare the volume of water in the weighing bottle from part 1 with the volume of water in the weighing bottle from part 2. Why are they different? show your numerical procedure: density of unknown metal show your numerical procedure: g/cm3 REPORT UNKNOWN METAL NUMBER AND DENSITY ON COVER SHEET. DID YOU REMEMBER TO SHOW ALL CALCULATIONS AND REPORT THE CORRECT NUMBER OF SIGNIFICANT FIGURES? unknown metal # 10 Questions 1. To how many significant figures must the volume of the weighing bottle be properly reported? answer 2. To how many significant figures must the density of the unknown liquid be properly reported? 3. To how many significant figures must the density of the unknown metal be properly reported? answer 4. Explain why the value obtained for the density of the metal has a higher inherent percentage of error than the value for the liquid. Hint: Include the mass and volume measurements of these two unknowns in your explanation answer ****ALL CALCULATIONS MUST BE SHOWNII||||**** Please do NOT use scientific notation or "e" values. Report values as decimals. 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


