Question: PLZ HELP!!! WITH THIS: Complete the LAB EXERCISE 4.5.1 on p. 278 (attached) Boiling Points and Intermolecular Forces. You may use either graphing software or
PLZ HELP!!! WITH THIS: Complete the LAB EXERCISE 4.5.1 on p. 278 (attached) Boiling Points and Intermolecular Forces. You may use either graphing software or graph paper for your graph. Turn in your work here when you are done.
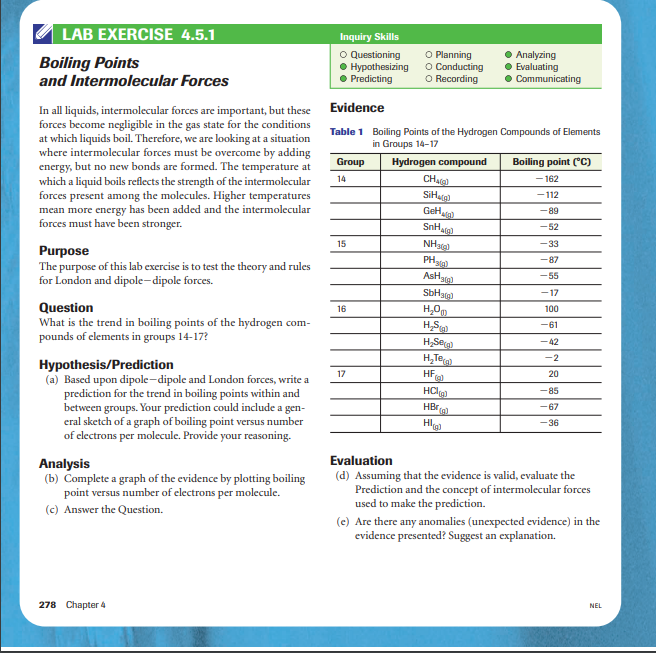
and Intermolecular Forces \begin{tabular}{|lll|} \hline Hypothesizing & O Conducting & Evaluating \\ Predicting & O Recording & Communicating \\ \hline \end{tabular} In all liquids, intermolecular forces are important, but these Evidence forces become negligible in the gas state for the conditions at which liquids boil. Therefore, we are looking at a situation Table 1 Boiling Points of the Hydrogen Compounds of Elements where intermolecular forces must be overcome by adding energy, but no new bonds are formed. The temperature at which a liquid boils reflects the strength of the intermolecular forces present among the molecules. Higher temperatures mean more energy has been added and the intermolecular forces must have been stronger. Purpose The purpose of this lab exercise is to test the theory and rules for London and dipole-dipole forces. Question What is the trend in boiling points of the hydrogen compounds of elements in groups 14-17? Hypothesis/Prediction (a) Based upon dipole-dipole and London forces, write a prediction for the trend in boiling points within and between groups. Your prediction could include a general sketch of a graph of boiling point versus number of electrons per molecule. Provide your reasoning. Analysis Evaluation (b) Complete a graph of the evidence by plotting boiling (d) Assuming that the evidence is valid, evaluate the point versus number of electrons per molecule. Prediction and the concept of intermolecular forces (c) Answer the Question. used to make the prediction. (c) Are there any anomalies (unexpected evidence) in the evidence presented? Suggest an explanation. and Intermolecular Forces \begin{tabular}{|lll|} \hline Hypothesizing & O Conducting & Evaluating \\ Predicting & O Recording & Communicating \\ \hline \end{tabular} In all liquids, intermolecular forces are important, but these Evidence forces become negligible in the gas state for the conditions at which liquids boil. Therefore, we are looking at a situation Table 1 Boiling Points of the Hydrogen Compounds of Elements where intermolecular forces must be overcome by adding energy, but no new bonds are formed. The temperature at which a liquid boils reflects the strength of the intermolecular forces present among the molecules. Higher temperatures mean more energy has been added and the intermolecular forces must have been stronger. Purpose The purpose of this lab exercise is to test the theory and rules for London and dipole-dipole forces. Question What is the trend in boiling points of the hydrogen compounds of elements in groups 14-17? Hypothesis/Prediction (a) Based upon dipole-dipole and London forces, write a prediction for the trend in boiling points within and between groups. Your prediction could include a general sketch of a graph of boiling point versus number of electrons per molecule. Provide your reasoning. Analysis Evaluation (b) Complete a graph of the evidence by plotting boiling (d) Assuming that the evidence is valid, evaluate the point versus number of electrons per molecule. Prediction and the concept of intermolecular forces (c) Answer the Question. used to make the prediction. (c) Are there any anomalies (unexpected evidence) in the evidence presented? Suggest an explanation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


