Question: Plz provide with python code! thanks! Q2. The semi-empirical mass formula ( 20 points) In modern physics course, you learnt/will learn the semi-empirical mass formula
Plz provide with python code! thanks!
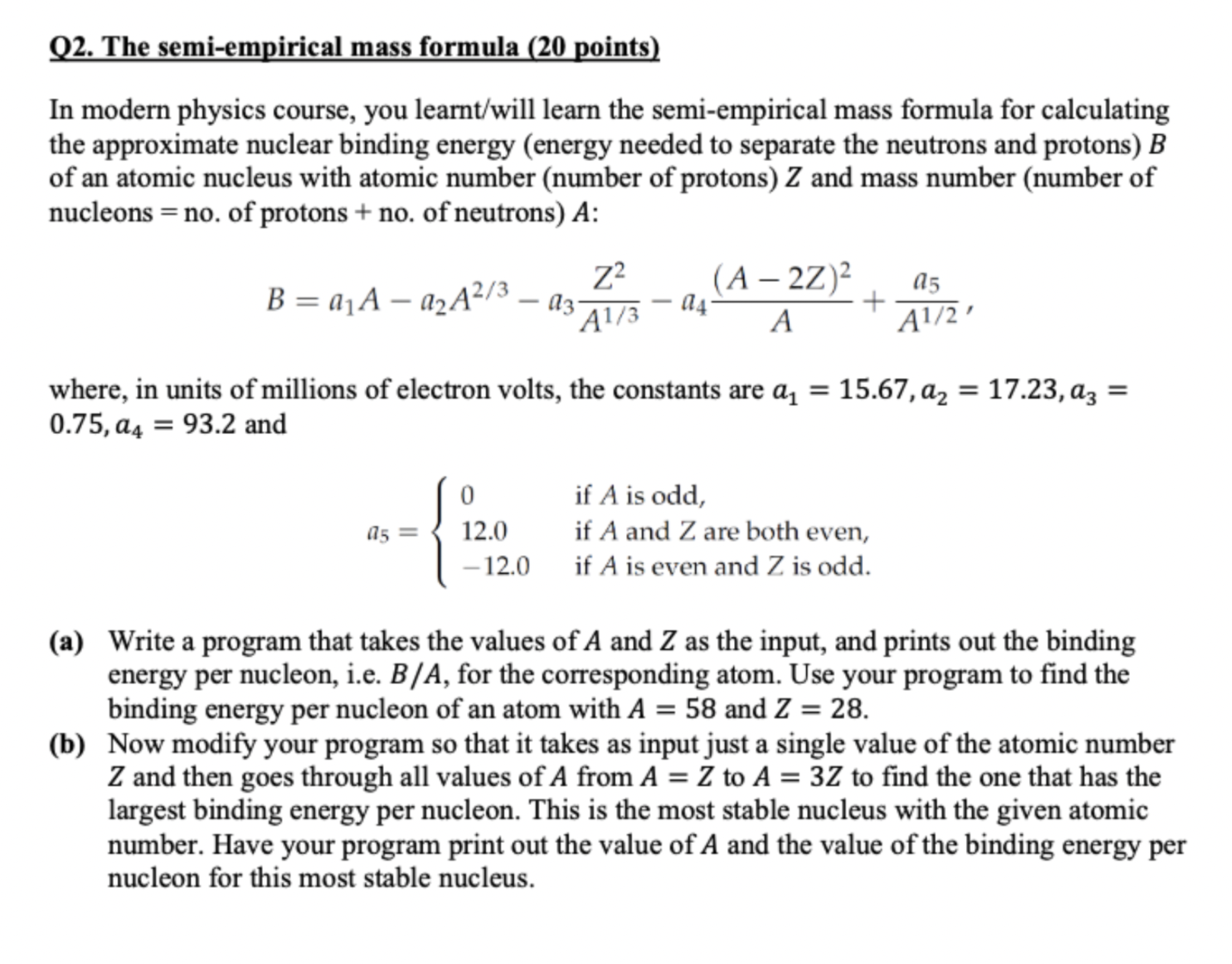
Q2. The semi-empirical mass formula ( 20 points) In modern physics course, you learnt/will learn the semi-empirical mass formula for calculating the approximate nuclear binding energy (energy needed to separate the neutrons and protons) B of an atomic nucleus with atomic number (number of protons) Z and mass number (number of nucleons = no. of protons + no. of neutrons) A : B=a1Aa2A2/3a3A1/3Z2a4A(A2Z)2+A1/2a5, where, in units of millions of electron volts, the constants are a1=15.67,a2=17.23,a3= 0.75,a4=93.2 and a5=012.012.0ifAisoddifAandZarebothevenifAisevenandZisodd (a) Write a program that takes the values of A and Z as the input, and prints out the binding energy per nucleon, i.e. B/A, for the corresponding atom. Use your program to find the binding energy per nucleon of an atom with A=58 and Z=28. (b) Now modify your program so that it takes as input just a single value of the atomic number Z and then goes through all values of A from A=Z to A=3Z to find the one that has the largest binding energy per nucleon. This is the most stable nucleus with the given atomic number. Have your program print out the value of A and the value of the binding energy per nucleon for this most stable nucleus
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


