Question: plz solve all question 9 & 10 & 3 all plz 9-a) Draw the Born Haber cycle for the formation of CsF(s). (Show all steps)
plz solve all question 9 & 10 & 3
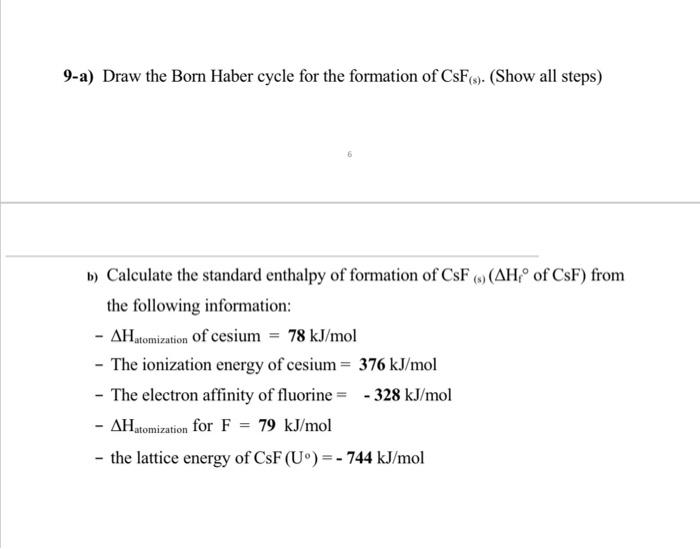
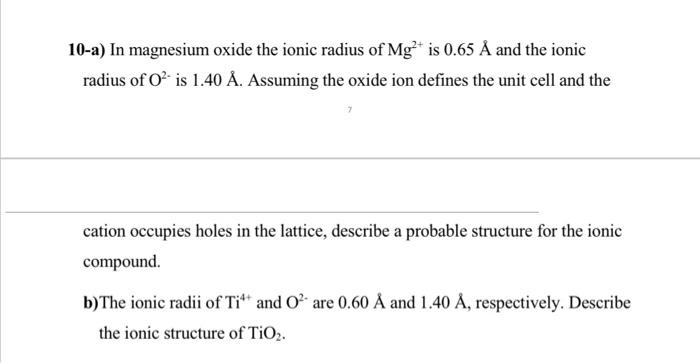

9-a) Draw the Born Haber cycle for the formation of CsF(s). (Show all steps) b) Calculate the standard enthalpy of formation of CsF(s)(Hf of CsF) from the following information: Hatomization of cesium =78kJ/mol - The ionization energy of cesium =376kJ/mol - The electron affinity of fluorine =328kJ/mol Hatomization for F=79kJ/mol - the lattice energy of CsF(U)=744kJ/mol 10-a) In magnesium oxide the ionic radius of Mg2+ is 0.65A and the ionic radius of O2 is 1.40A. Assuming the oxide ion defines the unit cell and the cation occupies holes in the lattice, describe a probable structure for the ionic compound. b) The ionic radii of Ti4+ and O2 are 0.60A and 1.40A, respectively. Describe the ionic structure of TiO2. 3-Arrange the following ions in order of decreasing size: Br,I,Mg2+,Ca2+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


