Question: Point Question 5 A 285 ml benzene solution containing 2.31 g of an organic polymer has an osmotic pressure of 8.33 mm Hg at 21C.
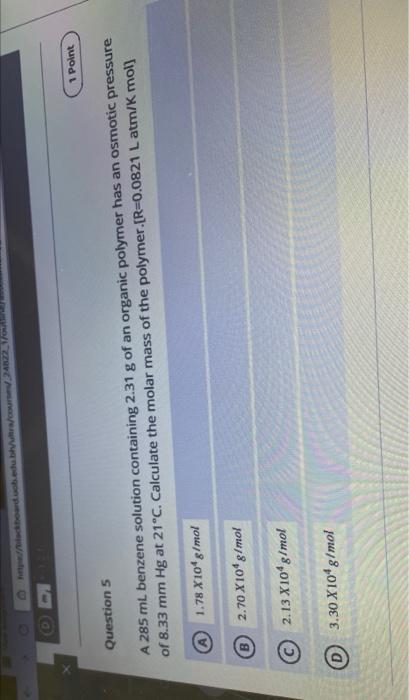
Point Question 5 A 285 ml benzene solution containing 2.31 g of an organic polymer has an osmotic pressure of 8.33 mm Hg at 21C. Calculate the molar mass of the polymer (R=0.0821 L atm/K mol] 1.78 X10* g/mol 2.70 X 109 g/mol B 2.13 X109 g/mol 0 3.30 X109 g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


