Question: poly math only poly math only (no hand writing) A reversible liquid-phase isomerization AB is carried out isothermally in a 1000-gal CSTR. The reaction is
 poly math only poly math only (no hand writing)
poly math only poly math only (no hand writing)
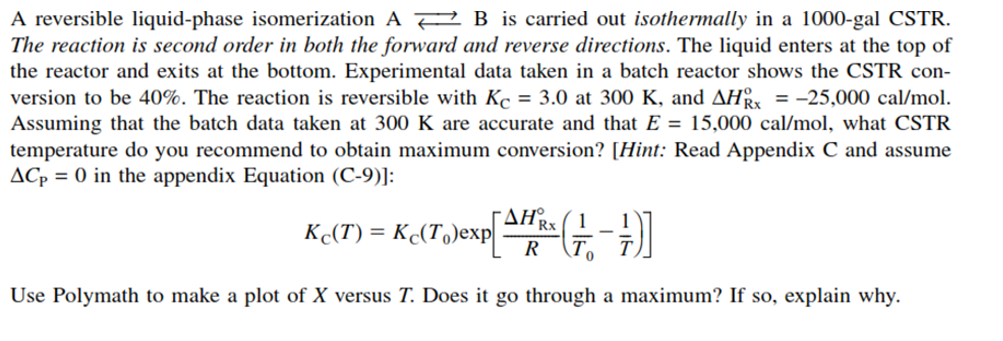
A reversible liquid-phase isomerization AB is carried out isothermally in a 1000-gal CSTR. The reaction is second order in both the forward and reverse directions. The liquid enters at the top of the reactor and exits at the bottom. Experimental data taken in a batch reactor shows the CSTR conversion to be 40%. The reaction is reversible with KC=3.0 at 300K, and HRxo=25,000cal/mol. Assuming that the batch data taken at 300K are accurate and that E=15,000cal/mol, what CSTR temperature do you recommend to obtain maximum conversion? [Hint: Read Appendix C and assume CP=0 in the appendix Equation (C-9)]: KC(T)=KC(T0)exp[RHRx(T01T1)] Use Polymath to make a plot of X versus T. Does it go through a maximum? If so, explain why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


