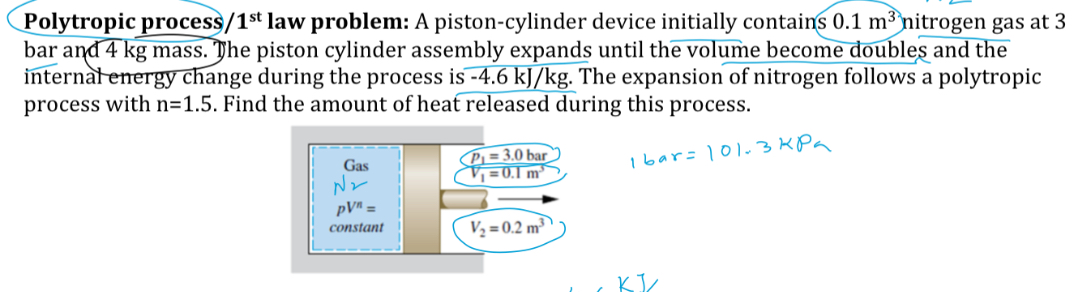
Question: Polytropic process / 1 1 s t law problem: A piston - cylinder device initially contains 0 . 1 m 3 nitrogen gas at 3
Polytropic process law problem: A pistoncylinder device initially contains nitrogen gas at bar and kg mass. The piston cylinder assembly expands until the volume become doubles and the internatenergy change during the process is The expansion of nitrogen follows a polytropic process with Find the amount of heat released during this process.
bar kPa
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


