Question: POST-LAB ASSIGNMENT FOR TECHNIQUE 27 - CARBON-13 NMR 10 questions at 10 pts, each = 100 points NAME: Type in your answers in the yellow
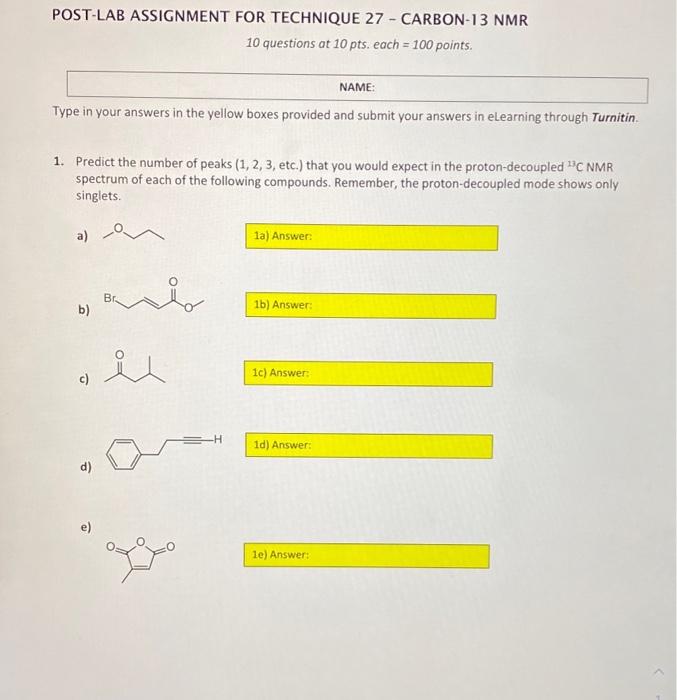
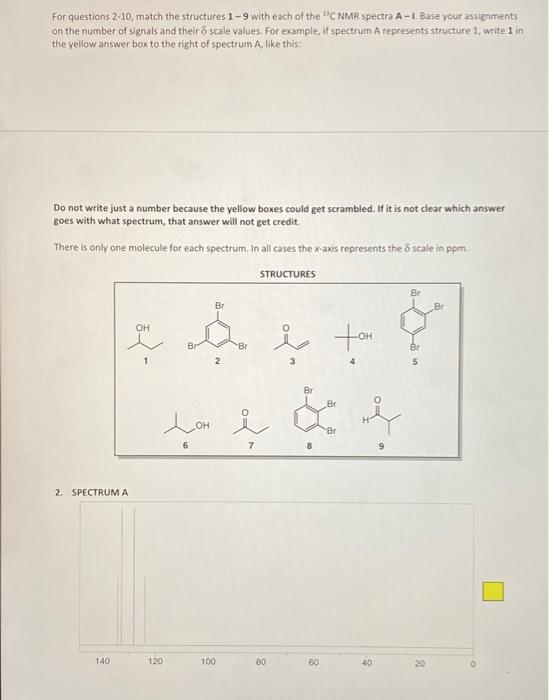
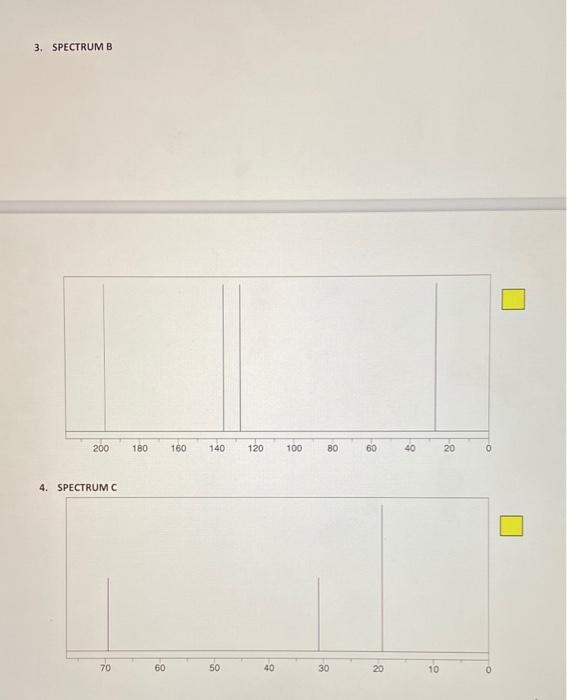
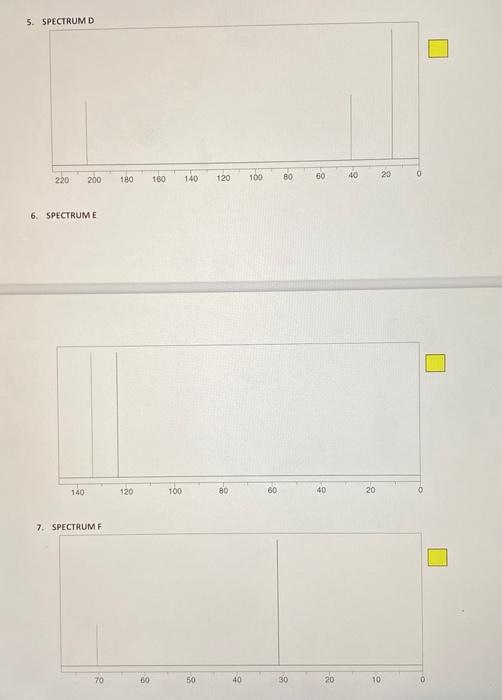
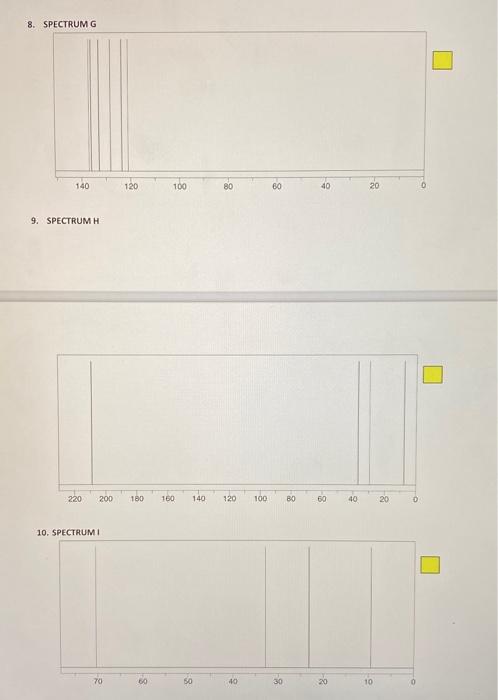
POST-LAB ASSIGNMENT FOR TECHNIQUE 27 - CARBON-13 NMR 10 questions at 10 pts, each = 100 points NAME: Type in your answers in the yellow boxes provided and submit your answers in eLearning through Turnitin 1. Predict the number of peaks (1, 2, 3, etc.) that you would expect in the proton-decoupled C NMR spectrum of each of the following compounds. Remember, the proton-decoupled mode shows only singlets. a) 1a) Answer: Br. b) 1b) Answer iu 1c) Answer: -H 1d) Answer: d) e) le) Answer: For questions 2-10, match the structures 1 - 9 with each of the "CNMR spectra A-1 Base your assignments on the number of signals and their 8 scale values. For example, if spectrum A represents structure 1, write 1 in the yellow answer box to the right of spectrum A, like this: Do not write just a number because the yellow boxes could get scrambled. If it is not clear which answer goes with what spectrum, that answer will not get credit There is only one molecule for each spectrum. In all cases the x-axis represents the scale in ppm STRUCTURES Br Br Br e ho ton i OH OH Br Br 5 1 2 4 Br BI Lor OH . 6 7 8 2. SPECTRUM A 140 120 100 80 80 40 20 3. SPECTRUM B . 200 180 160 140 120 100 80 60 40 20 4. SPECTRUM C 70 60 50 40 30 20 10 5. SPECTRUM D 200 0 220 140 60 180 120 40 160 100 20 80 6. SPECTRUME 140 120 100 80 60 10 20 7. SPECTRUMF 70 30 40 30 20 10 8. SPECTRUM G 140 120 100 80 80 40 20 0 9. SPECTRUMH 220 200 180 160 140 120 100 180) 60 40 20 0 10. SPECTRUMI - 170 40 20 10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


