Question: POTENTIOMETRY POSTLAB QUESTION Four tablet samples (1.6308 g) which contain acetylsalicylic acid were dissolved in a 100 ml water/ethanol mixture. A 25-ml aliquot was obtained
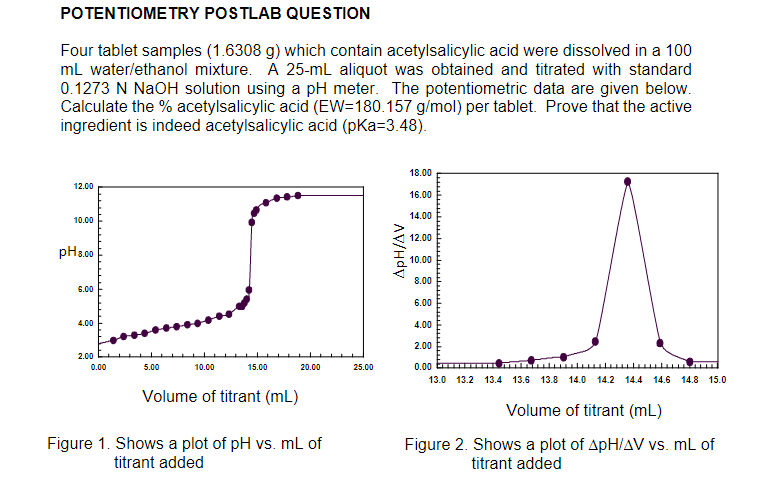
POTENTIOMETRY POSTLAB QUESTION Four tablet samples (1.6308 g) which contain acetylsalicylic acid were dissolved in a 100 ml water/ethanol mixture. A 25-ml aliquot was obtained and titrated with standard 0.1273 N NaOH solution using a pH meter. The potentiometric data are given below. Calculate the % acetylsalicylic acid (EW=180.157 g/mol) per tablet. Prove that the active ingredient is indeed acetylsalicylic acid (pka=3.48). 18.00 12.00 16.00 10.00 14.00 12.00 pH 8.00 ApH/AV 10.00 8.00 6.00 6.00 4.00 4.00 2.00 2.00 0.00 5.00 10.00 15.00 20.00 25.00 0.00 13.0 13.2 13.4 13.6 13.8 14.0 14.2 14.4 14.6 14.8 15.0 Volume of titrant (mL) Figure 1. Shows a plot of pH vs. mL of titrant added Volume of titrant (mL) Figure 2. Shows a plot of ApH/AV vs. mL of titrant added
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


