Question: Pow Miric 3 . 7 Che 1 ? ? courseld = 1 2 6 3 5 2 4 3 8 akey = 1 1 5
Pow
Miric
Che
courseld akey dots t
er Algorithmic Question
of
Constants Periodic T
Part A
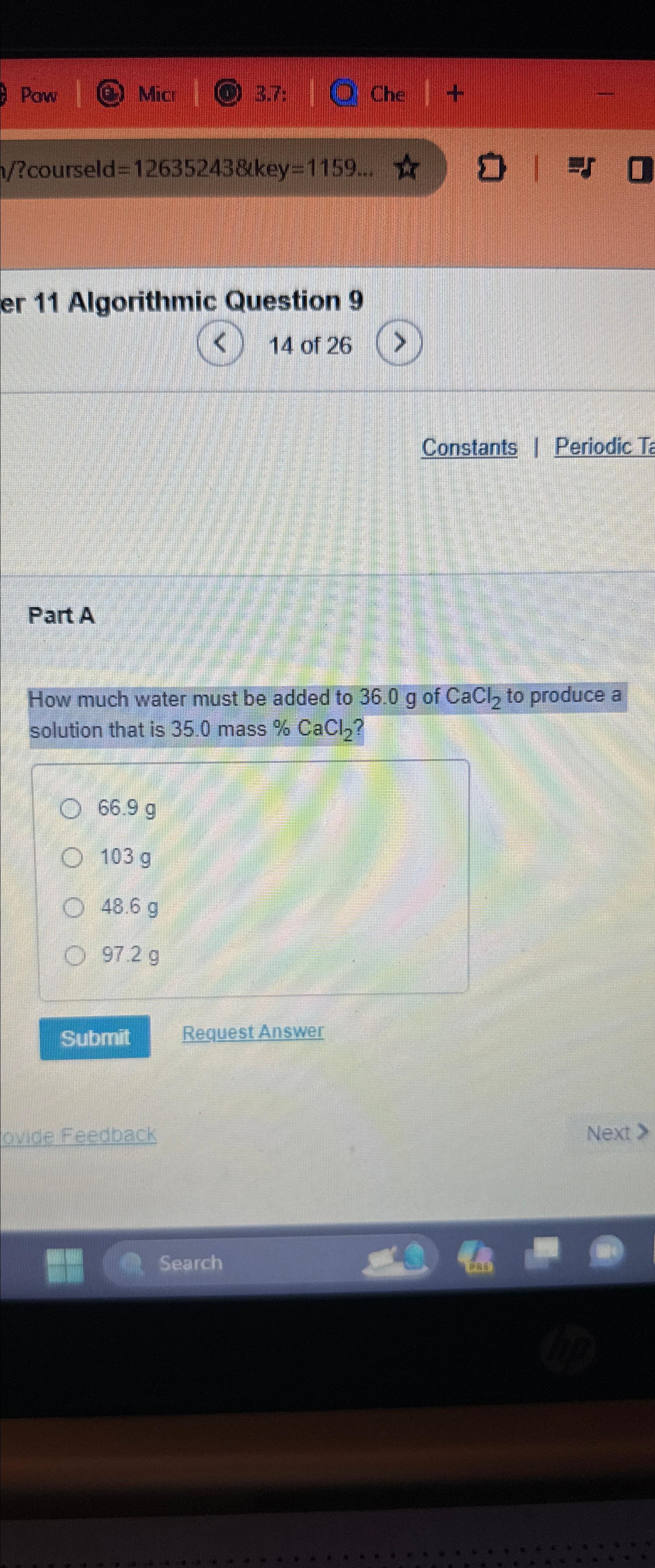
How much water must be added to of to produce a solution that is mass
ounce Feedback
Next
Search
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


