Question: pre lab questions please show the steps Pre-Lab Exercise 1. For reach reactant, calculate its initial concentration in the reactant mixture using the dilution formula:

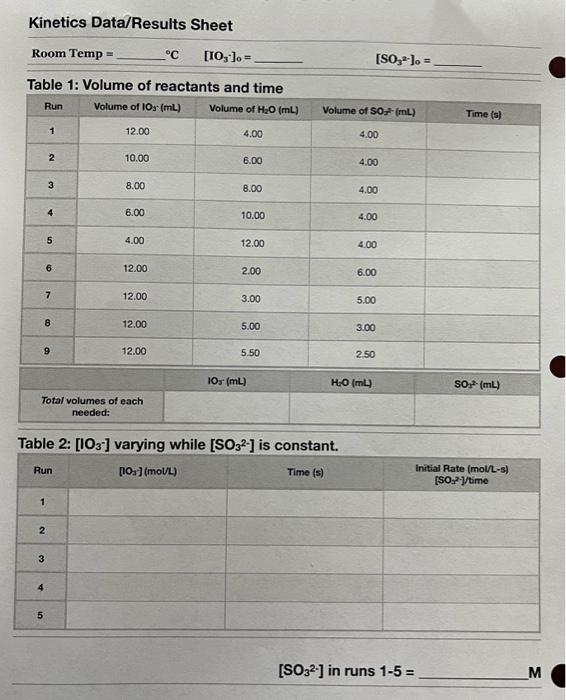


Pre-Lab Exercise 1. For reach reactant, calculate its initial concentration in the reactant mixture using the dilution formula: M1V1=M2V2 (Hint: M1 = the stock solution concentration. Also, consider in all cases that the reactant of original concentration has been diluted by other reactant and the distilled water for each run.) Show one detailed example of this calculation. 2. Calculate the total volume of each reagent needed. 3. Record the results of your calculations in the data table. Procedure You will be provided with two stock solutions, A and B. Solution A contains iodate ions, and solution B contains sulfite ions along with some acid and starch. Note the concentration of each stock solution on your data sheet. In preparing your reaction systems, always add water first, followed by solution B, then solution A. Calculate the total volume of each reagent needed and record it on your data sheet. Pour approximately that amount from the reagent bottle into an appropriate sized beaker. If you accidentally take too much, offer the extra to another group to minimize waste. For water, use distilled water. Use 10.00mL graduated cylinders to measure out the larger volumes and 5.00mL graduated pipets to measure out smaller volumes. Use a small (50 mL) beaker as your reaction vessel. Always add acid to water. As temperature changes affect reaction rates, you will want to minimize contact with the solutions. After combining water and solution B, begin stirring using a glass stirring rod while another group member quickly adds solution A. Make sure the stopwatch is started as soon as solution A is added. Time each reaction using the stopwatch and record the time in ink on your data sheet. Record the temperature of the room for the reactions. Table 2: [IO3]varying while [SO32] is constant. [SO32] in runs 15= M Method 2: Integrated Rate Law This method of determining reaction order is to plot experimental data of time of reaction versus a form of concentration ([A], 1/[A], In[A]) of the reactant on a graph. Of the three resulting graphs, the graph which is linear indicates the order of the reaction with respect to A. You can then determine the correct rate law (please refer to the table below). The Reaction to be Studied In this particular experiment you will study a reaction called the iodine clock reaction, in which aqueous solutions of sodium iodate and sodium sulfite react to form iodide and sulfate. The balanced chemical equation shows that 3 sulfite ions are required for every one iodate ion. Note: This makes the reaction appear to be a single collision between 4 different ions, but that will not happen in 1 step. The actual mechanism is a series of steps, each involving simple two-body collisions, which add up to give us the overall reaction. Reaction 1: IO5+3SO52F+3SO42= Since the reaction above has no endpoint, there is no way to know when to stop the stopwatch. A way around this dilemma is to use the following indicator system; we allow an excess of iodate ions to react with sulfite ions, when all of the sulfite ions (the limiting reagent) are consumed, the excess iodate reactant begins to react with the product iodide in the presence of aid (H+)to produce iodine molecules: Reaction 2: 1O3+5I+6H+3I2+3H2O The iodine molecules produced ean be detected by the addition of starch as they form a dark blue complex: Reaction 3: starch +I2 starch-iodine complex The rate of this reaction can be determined by measuring the time it takes for the blue color to appear. Although the reaction is a complex one, it can be studied rather easily because of the "rate-limiting" step concept in Kinetics. According to this concept, the slowest step in any multi-step reaction determines the overall rate of the reaction. The slow step in this reaction has been found to be Reaction 1 , and the other reactions can be assumed to occur instantaneously as soon as Il of the sulfite ions are consumed. Hence, the rate expression for this reaction is that expression which describes the slowest step. Rate=k[iodate]m[sulfite]D Measurement of Rate of Reaction In this experiment, the rate of the reaction will be based on how long it takes for the blue color of the starch-iodine complex to appear. We will assume that when the blue color appears, all of the sulfite ions have been consumed, SO32 - is the limiting reagent and [SO32-] ] final =0. The rate of the reaction will be expressed as the change in sulfite concentration over time, or: Rate=[SO32]/time=[SO32]2]iritial/time For simplicity, the word "initial" will be omitted for the rest of the lab. Iodate is an excess reagent, it is not correct to say that the change in concentration is the same as the initial concentration. Potential Hazards In the event of an accident, immediately inform an instructor. Hazard: Potassium iodate and sodium sulfite may be harmful if swallowed, and may cause irritation to skin and eyes. The sodium sulfite solution is acidic. Precaution: Wear goggles, always add acid to water, wash off any acid that might be outside the acid bottle. Action: Thoroughly rinse with water, use safety shower if necessary. Use sodium bicarbonate solution to neutralize the acid. Go to the nurse for treatment if necessary. Use eyewash if acid is splashed into the eye
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


