Question: Predict how many emission lines will be seen by a human in the visible spectrum of atomic hydrogen. (Assume that the visible spectrum ranges from
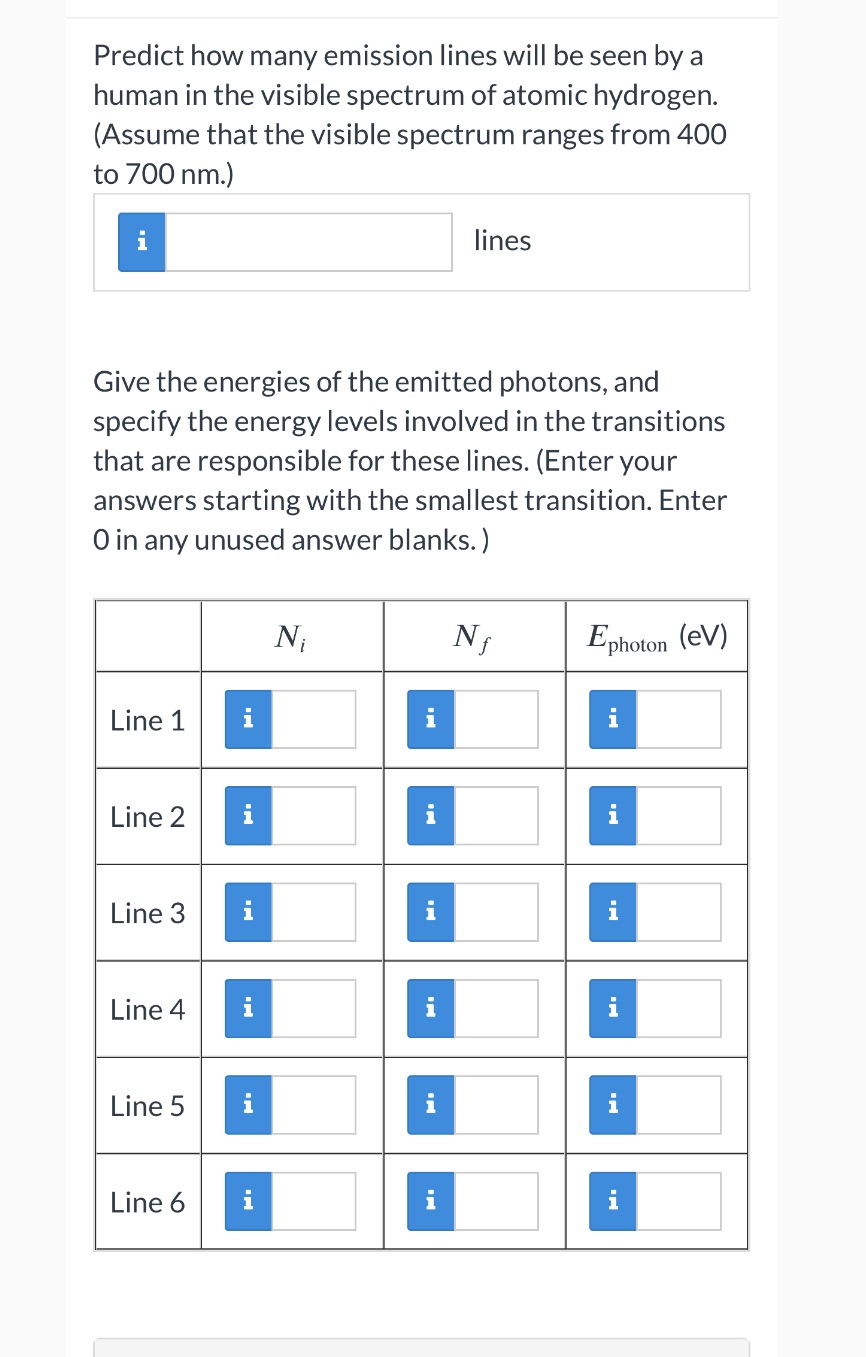
Predict how many emission lines will be seen by a human in the visible spectrum of atomic hydrogen. (Assume that the visible spectrum ranges from 400 to 700 nm.) a lines Give the energies of the emitted photons, and specify the energy levels involved in the transitions that are responsible for these lines. (Enter your answers starting with the smallest transition. Enter 0 in any unused answer blanks.) N ,- Nf Ephom (eV) Line 1 I I a Line 2 n I a Line 3 n l a Line 4 n I a Line 5 l l a Line 6 n I n
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


