Question: pre-lab calculations please show work Part 1 - Making solutions The following solutions should be made, you will need a total of 8 vials to

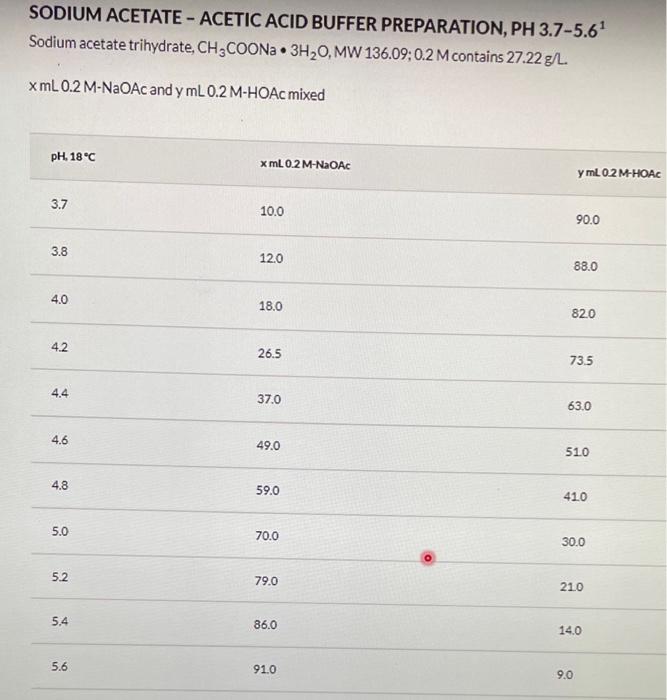
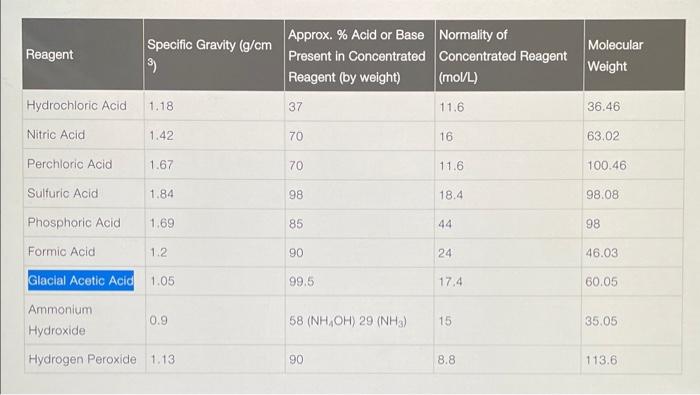
Part 1 - Making solutions The following solutions should be made, you will need a total of 8 vials to make the solutions: - Acetate buffer ( 0.2MpH4.65) : - 0.2M sodium acetate (NaCH3COO) in 100mL of DI water - 0.2M acetic acid (CH3COO) in 100mL of DI water - You would make a dilution from the concentrated acid (Concentration of Common Acids/Bases (Concentrated)) Determine the amount of each to mix to make pH4.6 in 100mL total - Use the Buffer Reference Center. - Pb2+ solutions: 0.1M nitric acid (HNO3) in 50mL DI water. Use the link above HNO3 in 10mL. Pb2+ in 12mL of the 0.2MpH4.6 acetate buffer. SODIUM ACETATE - ACETIC ACID BUFFER PREPARATION, PH 3.75.61 Sodium acetate trihydrate, CH3COONa3H2O,MW136.09;0.2 Mcontains 27.22g/L xmL0.2MNaOAc and mmL0.2MHOAcmixed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


