Question: Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-equivalent hydrogens in the immediate vicinity of the hydrogen
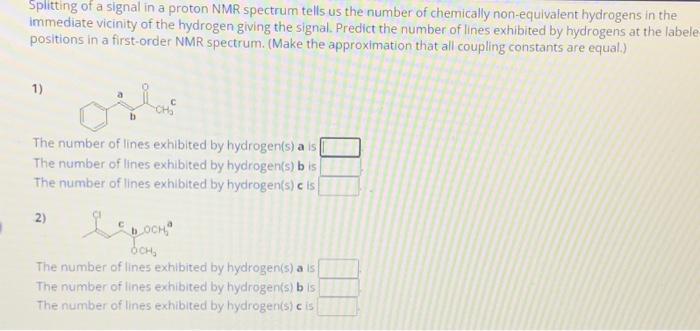
Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labele positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 1) CH The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is 2) The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is. The number of lines exhibited by hydrogen(s) c is
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Problem Breakdown The question involves interpreting a proton NMR spectrum where the splitting of a signal depends on the number of chemically nonequi... View full answer

Get step-by-step solutions from verified subject matter experts


