Question: Preparing a given volume of a solution that has a specific molarity is a very important skil for a chemist. One step in that process
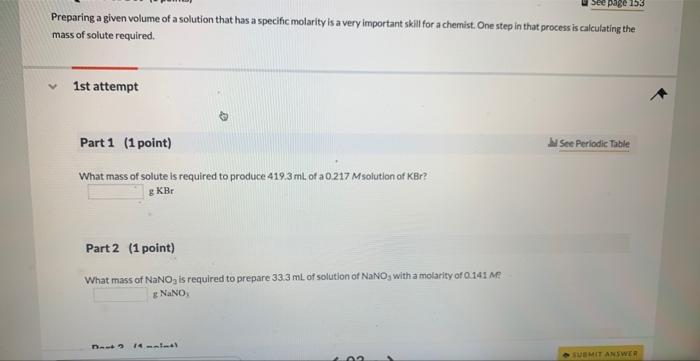
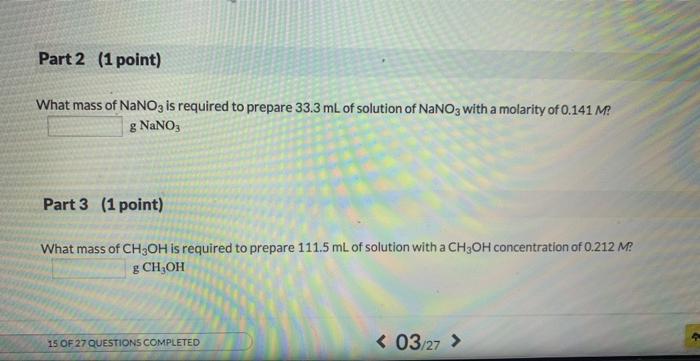
Preparing a given volume of a solution that has a specific molarity is a very important skil for a chemist. One step in that process is calculating the mass of solute required. 1st attempt Part 1 (1 point) Mee Periodic Table What mass of solute is required to produce 419.3mL of a 0.217Msolution of KBr ? gKBr Part 2 (1 point) What mass of NaNO2 is required to prepare 33.3mL of solution of NaNO3 with a molarity of Q141Me : g NaNO3 What mass of NaNO3 is required to prepare 33.3mL of solution of NaNO3 with a molarity of 0.141M ? gNaNO3 Part 3 (1 point) What mass of CH3OH is required to prepare 111.5mL of solution with a CH3OH concentration of 0.212M ? gCH3OH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


