Question: Previous answer of 52.79 was wrong (marked in red). please give correct answer. Thanks so much. Score on last try: 0.5 of 1 pts. See
Previous answer of 52.79 was wrong (marked in red). please give correct answer. Thanks so much. 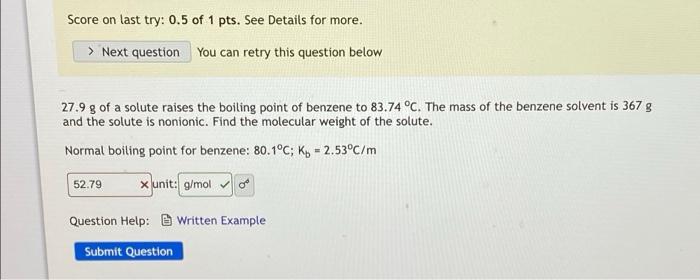

Score on last try: 0.5 of 1 pts. See Details for more. > Next question You can retry this question below 27.9 g of a solute raises the boiling point of benzene to 83.74 C. The mass of the benzene solvent is 367 g and the solute is nonionic. Find the molecular weight of the solute. Normal boiling point for benzene: 80.1C; Ko = 2.53C/m 52.79 x unit: g/mol Question Help: Written Example Submit
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


