Question: Problem 07.122 - Final equilibrium temperature and entropy change in insulated and sealed room. One ton of liquid water at 80C is brought into
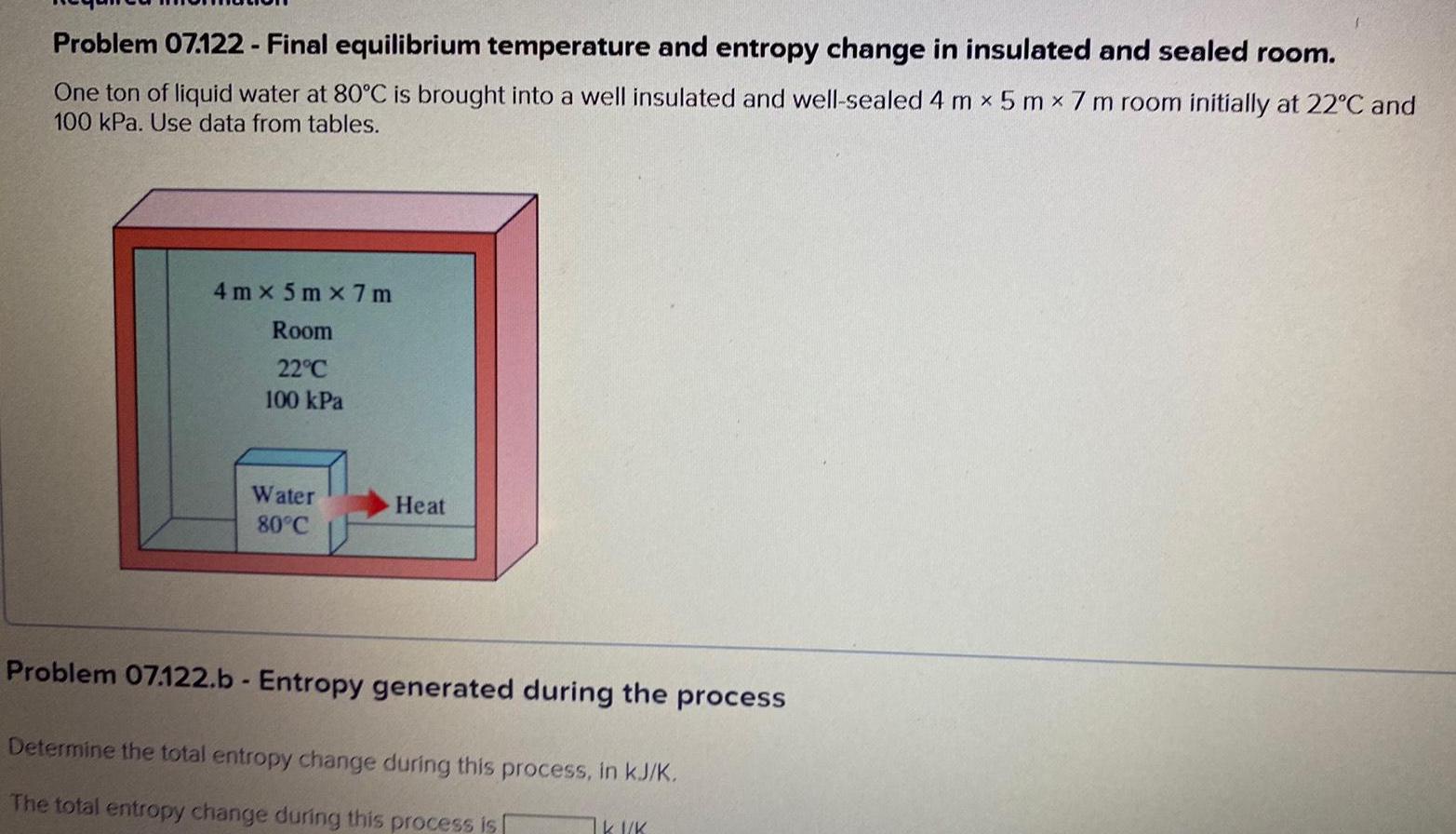
Problem 07.122 - Final equilibrium temperature and entropy change in insulated and sealed room. One ton of liquid water at 80C is brought into a well insulated and well-sealed 4 m 5 mx 7 m room initially at 22C and 100 kPa. Use data from tables. 4m x 5m x 7m Room 22C 100 kPa Water 80C Heat Problem 07.122.b - Entropy generated during the process Determine the total entropy change during this process, in kJ/K. The total entropy change during this process is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


