Question: Problem 1 ( 1 0 p t s ) Enthalpy is an important thermodynamic property of a system. Technically, it is the sum of the
Problem
Enthalpy is an important thermodynamic property of a system. Technically, it is the sum of the
internal energy of the system plus the product of the pressure times the volume of the system.
However, since the pressure times volume term is small for most systems especially solids and
liquids the enthalpy is often used as a standin for the internal energy of the system. While it is
not possible to measure the internal energy of the system, we can measure the change in enthalpy
of the with respect to that of a reference system.
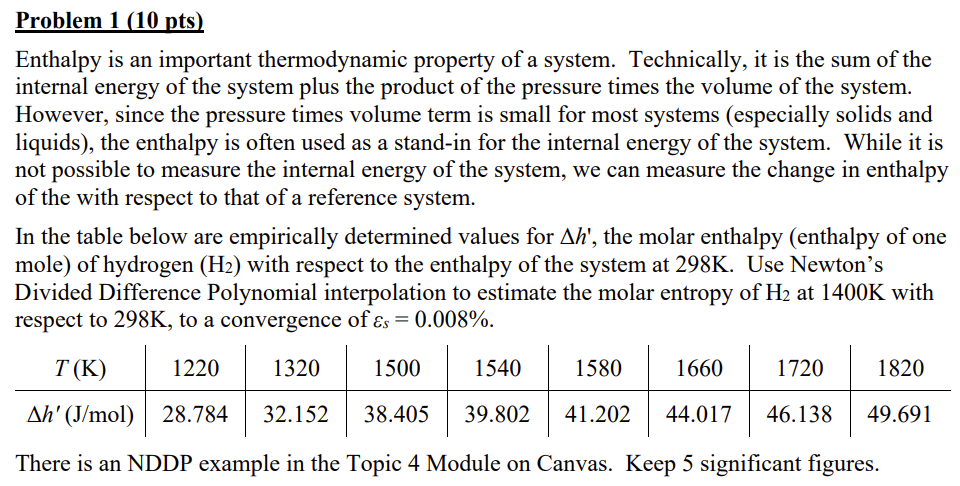
In the table below are empirically determined values for the molar enthalpy enthalpy of one
mole of hydrogen with respect to the enthalpy of the system at K Use Newton's
Divided Difference Polynomial interpolation to estimate the molar entropy of at K with
respect to K to a convergence of
There is an NDDP example in the Topic Module on Canvas. Keep significant figures.Problem
Enthalpy is an important thermodynamic property of a system. Technically, it is the sum of the
internal energy of the system plus the product of the pressure times the volume of the system.
However, since the pressure times volume term is small for most systems especially solids and
liquids the enthalpy is often used as a standin for the internal energy of the system. While it is
not possible to measure the internal energy of the system, we can measure the change in enthalpy
of the with respect to that of a reference system.
In the table below are empirically determined values for the molar enthalpy enthalpy of one
mole of hydrogen with respect to the enthalpy of the system at K Use Newton's
Divided Difference Polynomial interpolation to estimate the molar entropy of at K with
respect to K to a convergence of
There is an NDDP example in the Topic Module on Canvas. Keep significant figures.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


