Question: Problem 1: (15 points) In reference to the HDA process for production of benzene from toluene, answer the following questions: a) Justify the reactor inlet
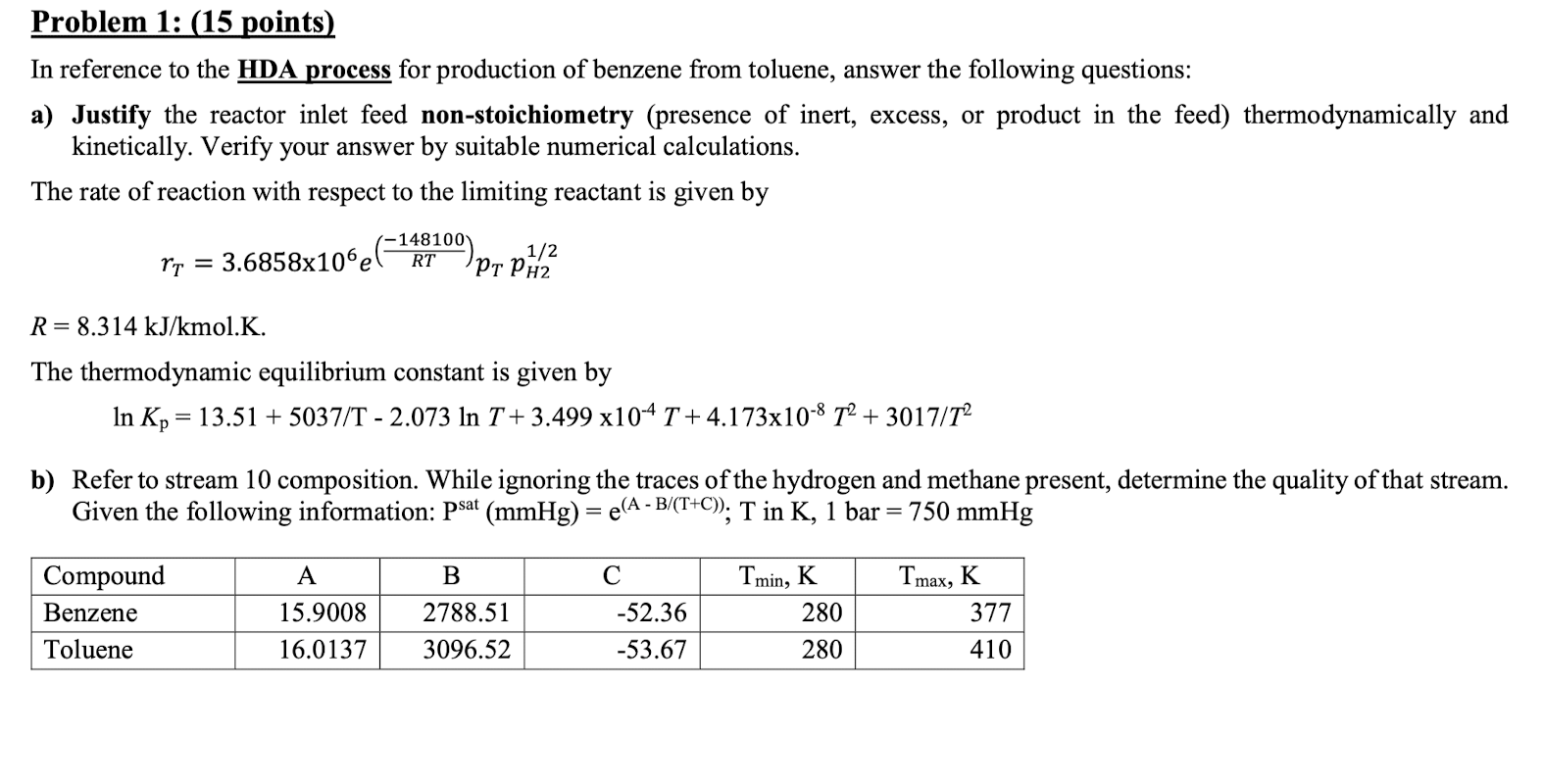
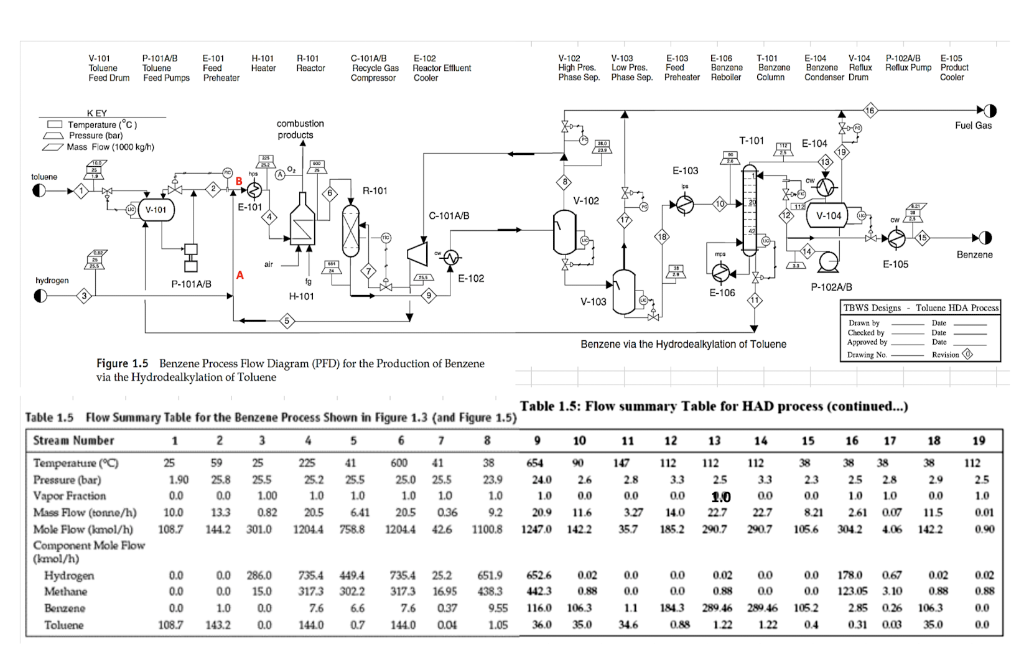
Problem 1: (15 points) In reference to the HDA process for production of benzene from toluene, answer the following questions: a) Justify the reactor inlet feed non-stoichiometry (presence of inert, excess, or product in the feed) thermodynamically and kinetically. Verify your answer by suitable numerical calculations. The rate of reaction with respect to the limiting reactant is given by 3.6858x106e -148100) 1/2 PH2 rt = RT R= 8.314 kJ/kmol.K. The thermodynamic equilibrium constant is given by In Kp = 13.51 + 5037/T - 2.073 In T+3.499 x104 T +4.173x10-8 T2 + 3017/T2 b) Refer to stream 10 composition. While ignoring the traces of the hydrogen and methane present, determine the quality of that stream. Given the following information: psat (mmHg) = e(A - B/(T+C); T in K, 1 bar = 750 mmHg = 2 A B Compound Benzene Toluene Tmin, K 280 Tmax, K 377 15.9008 16.0137 2788.51 3096.52 -52.36 -53.67 280 410 V-101 P-101A/B Toluene Toluene Feed Drum Feed Pumps E-101 Feed Preheater H-101 Heater R-101 Reactor C-101A/B E-102 Recycle Gas Reactor Effluent Compressor Cooler V-102 V-103 E-103 E-106 High Pres . Low Pres. Food Benzone Phase Sep. Phase Sep. Phase Sep. Preheater Reboiler T-101 Banzone Column E-104 V-104 Benzene Reflux Condenser Drum P-102A/B E-105 Reflux Pump Product Cooler Fuel Gas KEY O Temperature (C) Pressure (bar) Mass Flow (1000 kg/h) combustion products T-101 E-104 E-103 toluene 1 R-101 V-102 (V V-101 E-101 C-101A/B V-104) 18 U Benzene alr E-105 hydrogen E-102 P-101A/B 1g H-101 E-106 V-103 Toluene HDA Process P-102A/B P- TBWS Designs Drawn by Checked by Approved by Drawing No. Benzene via the Hydrodealkylation of Toluene Date Date Dwie Revision Figure 1.5 Benzene Process Flow Diagram (PFD) for the Production of Benzene via the Hydrodealkylation of Toluene 1.0 Table 1.5 Flow Summary Table for the Benzene Process Shown in Figure 1.3 (and Figure 1.5) Table 1.5: Flow summary Table for HAD process (continued...) Stream Number 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Temperature (C) 25 59 25 225 41 600 41 38 654 90 147 112 112 112 38 38 38 38 112 Pressure (bar) 1.90 25.8 25.5 25.2 25.5 25.0 25.5 23.9 24.0 26 28 3.3 25 33 23 25 28 29 25 Vapor Fraction 0.0 0.0 1.00 1.0 1.0 1.0 1.0 1.0 10 0.0 00 0.0 00 0.0 10 10 0.0 1.0 Mass Flow (tonne/h) 10.0 0.82 20.5 6.41 20.5 0.36 9.2. 20.9 11.6 3.27 14.0 22.7 227 8.21 2.61 0.07 115 0.01 Mole Flow (kmol/h) 108.7 144.2 301.0 1204.4 758.8 1204.4 426 1100.8 1247.0 1422 357 185.2 290.7 290.7 105.6 304.2 4.06 1422 0.90 Component Mole Flow (kmol/h) ( 0.0 0.0 286.0 735.4 449.4 735.4 25.2 651.9 6526 0.02 0.0 0.0 0.02 00 0.0 178.0 0.67 0.02 0.02 Methane 0.0 0.0 15.0 3173 3022 3173 16.95 438.3 442 3 0.88 0.0 0.0 0.88 0.0 0.0 123.05 3.10 0.88 0.88 Benzene 0.0 1.0 0.0 7.6 6.6 7.6 0.37 9.55 116.0 106.3 1.1 1843 289.46 289.46 1052 2.85 0.26 1063 00 Toluene 108.7 143.2 0.0 144.0 0.7 144.0 0.04 1.05 36.0 35.0 34.6 0.89 1.22 1.22 04 0.31 0.03 35.0 0.0 13.3 Hydrogen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


