Question: Problem 1 ( 2 0 points ) For a non - ideal gas obeying the Van der Waals equation ( P + a ? b
Problem points
For a nonideal gas obeying the Van der Waals equation
Using the fact that at the critical point must be
and
Prove the following relations between the constants a e b and the critical pressure and
temperature
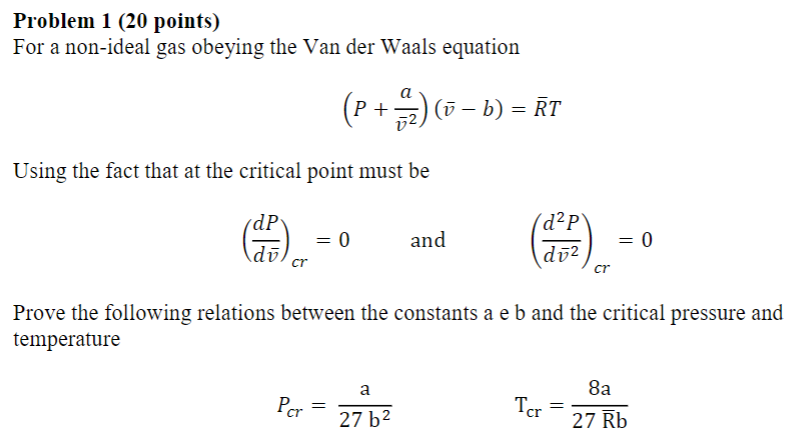
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


