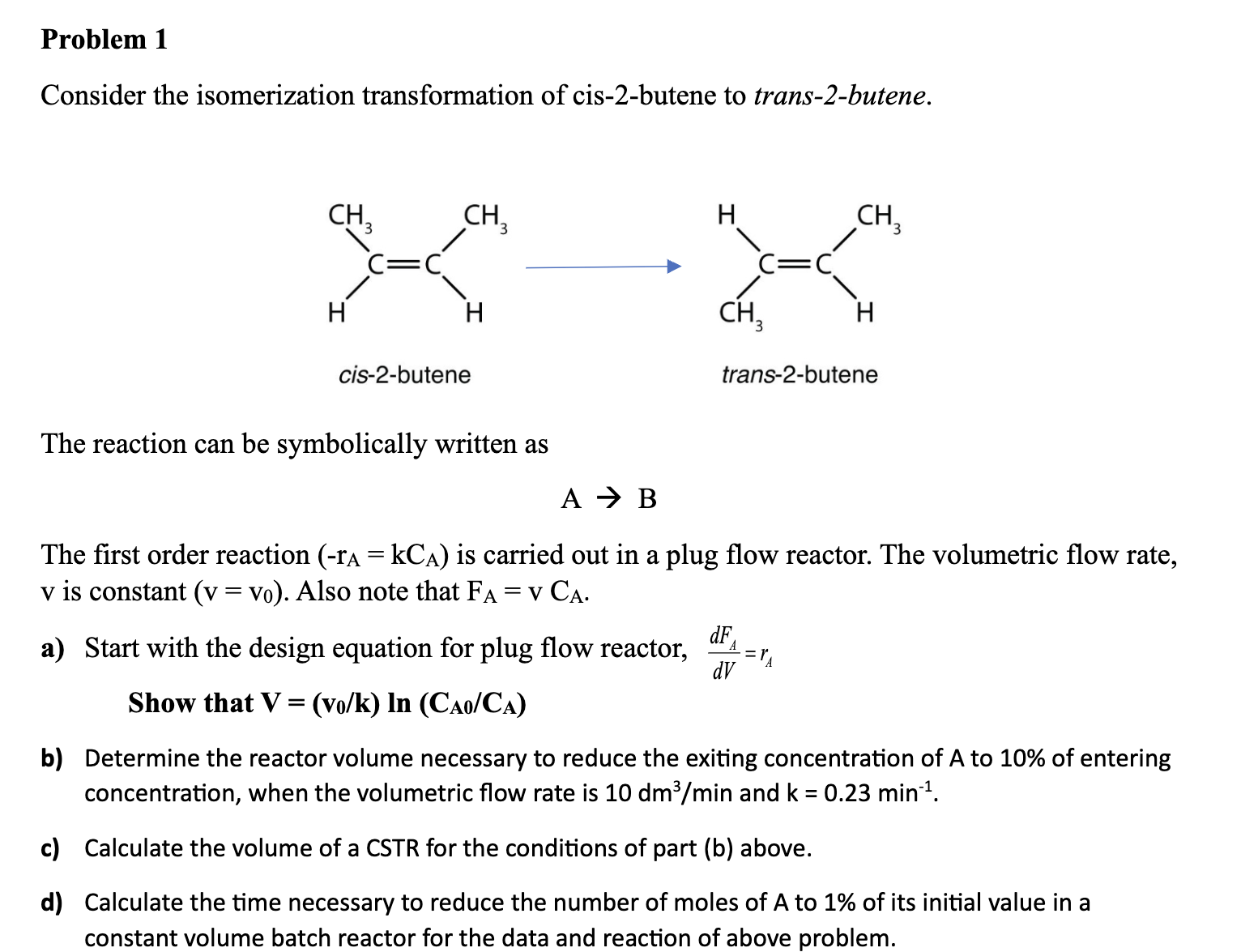
Question: Problem 1 Consider the isomerization transformation of cis - 2 - butene to trans - 2 - butene. The reaction can be symbolically written as
Problem
Consider the isomerization transformation of cisbutene to transbutene.
The reaction can be symbolically written as
The first order reaction is carried out in a plug flow reactor. The volumetric flow rate,
is constant Also note that
a Start with the design equation for plug flow reactor,
Show that
b Determine the reactor volume necessary to reduce the exiting concentration of to of entering
concentration, when the volumetric flow rate is and
c Calculate the volume of a CSTR for the conditions of part b above.
d Calculate the time necessary to reduce the number of moles of to of its initial value in a
constant volume batch reactor for the data and reaction of above problem.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


