Question: Problem 1. Determine the rate equation (from Shuler) E+Sk4k1(ES)k2k2E+P Develop a rate expression for product-formation using the quasi-steady-state approximation and show that v=dtd[P]=1+Km[S]+Kp[P](vz/Km)[S](vp/Kp)[P] where Km=k1k1+k2
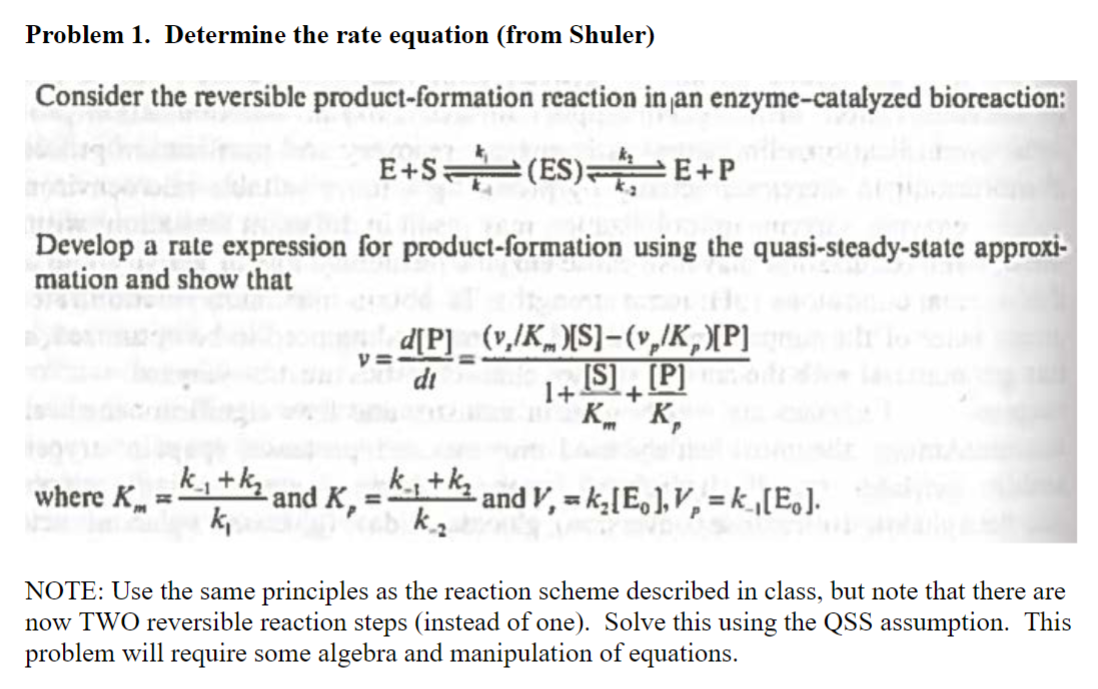
Problem 1. Determine the rate equation (from Shuler) E+Sk4k1(ES)k2k2E+P Develop a rate expression for product-formation using the quasi-steady-state approximation and show that v=dtd[P]=1+Km[S]+Kp[P](vz/Km)[S](vp/Kp)[P] where Km=k1k1+k2 and Kp=k2k1+k2 and V,=k2[E0],Vp=k1[E0] NOTE: Use the same principles as the reaction scheme described in class, but note that there are now TWO reversible reaction steps (instead of one). Solve this using the QSS assumption. This problem will require some algebra and manipulation of equations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


