Question: Problem 1. Heating Solid Carbon (30 points) A sample of carbon in the solid phase at 31.2C is heated to 77.4C in a continuous process
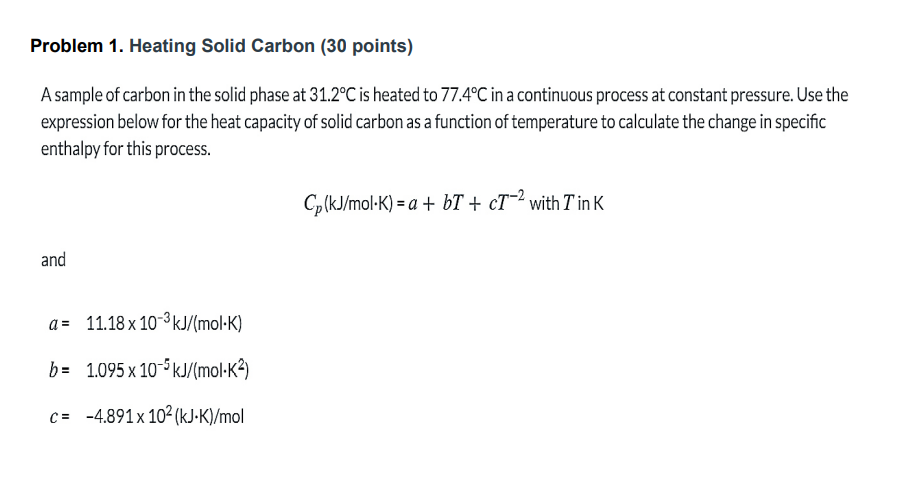
Problem 1. Heating Solid Carbon (30 points) A sample of carbon in the solid phase at 31.2C is heated to 77.4C in a continuous process at constant pressure. Use the expression below for the heat capacity of solid carbon as a function of temperature to calculate the change in specific enthalpy for this process. Cp(kJ/molK) = a + b+ ct-2 with Tin K and a = 11.18 x 10-3kJ/(mol-K) b= 1.095 x 10-5 kJ/(mol-K?) C= -4.891x 102(kJK)/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


