Question: Problem 1 Hydrogen atom a) An electron in a hydrogen atom starts out in the linear combination of stationary states: 1 t 11(1': U) $09100
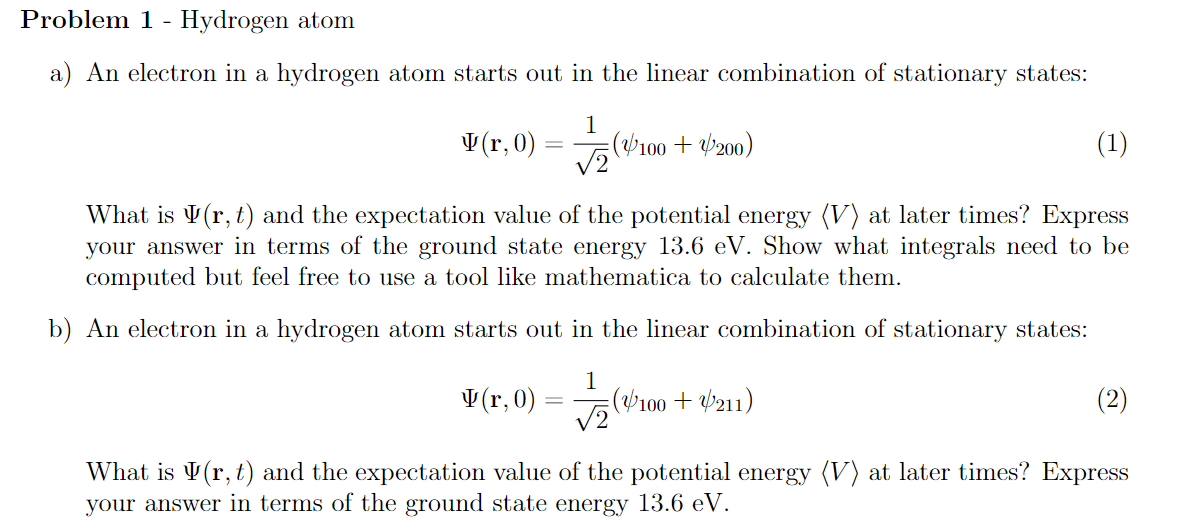
Problem 1 Hydrogen atom a) An electron in a hydrogen atom starts out in the linear combination of stationary states: 1 t 11"(1': U) $09100 + 11.9200) (1) \"That is \\lltrj L] and the expectation value of the potential energy (V) at later times? Express your answer in terms of the ground state energy 13.6 eV' Show what. integrals need to be computed but feel free to use a tool like mathematica to calculate them. 1)) An electron in a hydrogen atom starts out in the linear combination of stationary states: 1 ' \\Il(r: U) E (Winn + 1-9211) (2) 'Nhat is 111{r: L) and the expectation value of the potential energy (V) at later times? Express your answer in terms of the ground state energy 13.6 eV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


