Question: Problem 1 . It can be shown using the Maxwell relations of thermodynamics that the difference of specific heats can be represented as C p
Problem It can be shown using the Maxwell relations of thermodynamics that the difference
of specific heats can be represented as
The volume expansivity and the isothermal compressibility can be expressed as
Show that the specific heat difference can be written as
Also, use the above relation to show that the difference between the specific heats for an ideal
gas is the gas constant R Show all steps in your work for this problem.
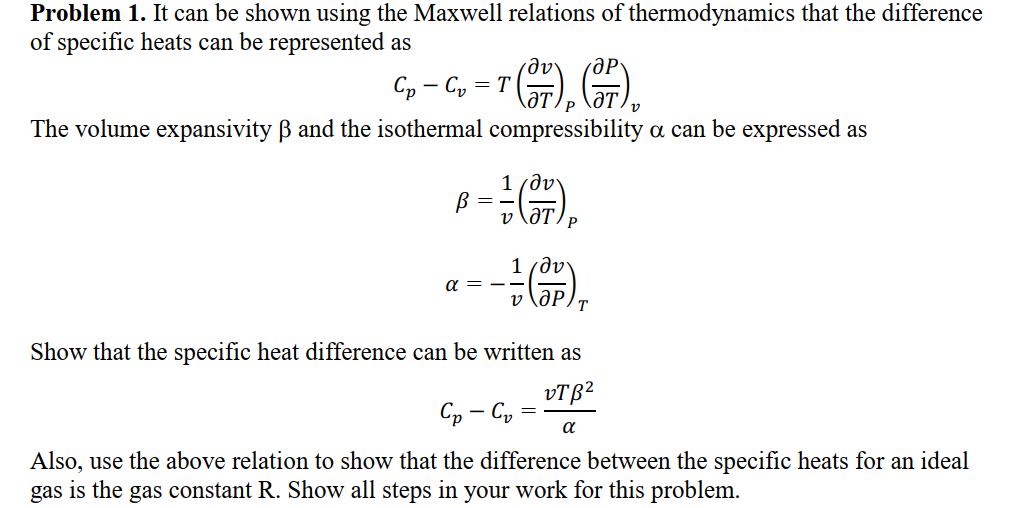
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


