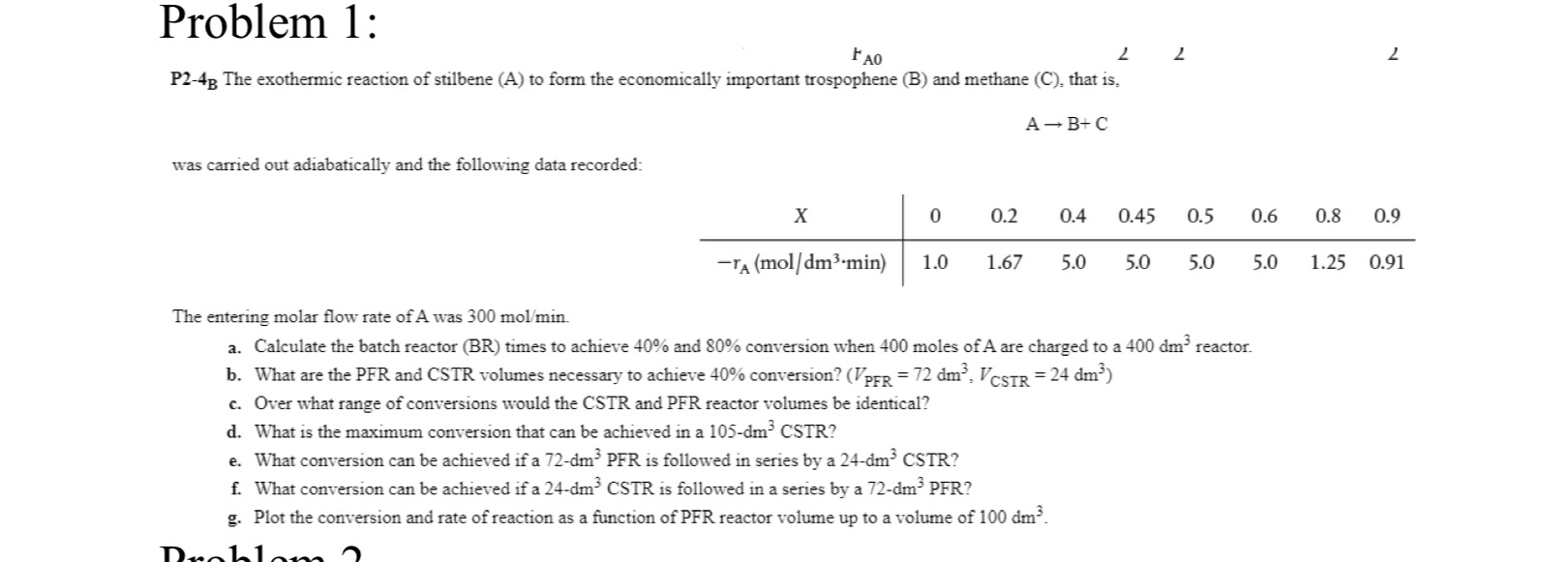
Question: Problem 1 : r A 0 2 P 2 - 4 The exothermic reaction of stilbene ( A ) to form the economically important trospophene
Problem :
P The exothermic reaction of stilbene A to form the economically important trospophene B and methane C that is
was carried out adiabatically and the following data recorded:
table
The entering molar flow rate of A was
a Calculate the batch reactor BR times to achieve and conversion when moles of A are charged to a reactor.
b What are the PFR and CSTR volumes necessary to achieve conversion?
c Over what range of conversions would the CSTR and PFR reactor volumes be identical?
d What is the maximum conversion that can be achieved in a
e What conversion can be achieved if a PFR is followed in series by a
f What conversion can be achieved if a is followed in a series by a
g Plot the conversion and rate of reaction as a function of PFR reactor volume up to a volume of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


