Question: Problem 1: The Hydrogen Atom. (20 points) (a) For 1s orbital of the hydrogen atom, the quantum mechanical solution is 1 V18 = 1/2
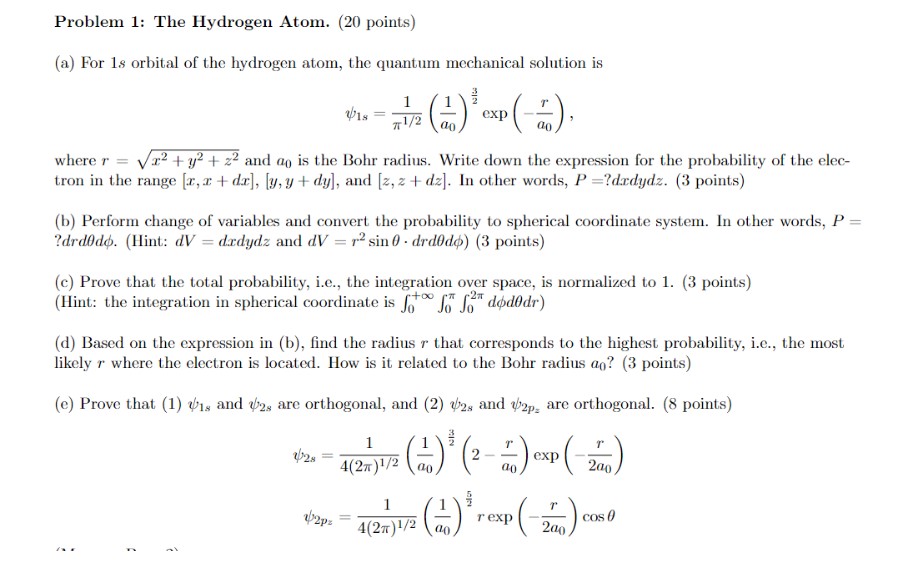
Problem 1: The Hydrogen Atom. (20 points) (a) For 1s orbital of the hydrogen atom, the quantum mechanical solution is 1 V18 = 1/2 an exp ao where r =2+ y+z2 and ao is the Bohr radius. Write down the expression for the probability of the elec- tron in the range [x, x+dx], [y, y+dy], and [z, z+dz]. In other words, P=?dadydz. (3 points) (b) Perform change of variables and convert the probability to spherical coordinate system. In other words, P = ?drdodo. (Hint: dV = drdydz and dV = r sin 0 drd0do) (3 points) (c) Prove that the total probability, i.e., the integration over space, is normalized to 1. (3 points) (Hint: the integration in spherical coordinate is fofododdr) (d) Based on the expression in (b), find the radius r that corresponds to the highest probability, i.e., the most likely r where the electron is located. How is it related to the Bohr radius ao? (3 points) (e) Prove that (1) 1s and 2s are orthogonal, and (2) 2s and 2p, are orthogonal. (8 points) 1 128 4(2)1/200 1 v2pz = (2-7) exp(-200) (+1) rexp (-200) 4(2) 1/2 ao cos 0 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


