Question: Problem 1(10 points): The value for R is 8.314 x 10'3 kJ/mole K(fpr this value, K means Kelvin). The following questions are based on hypothetical
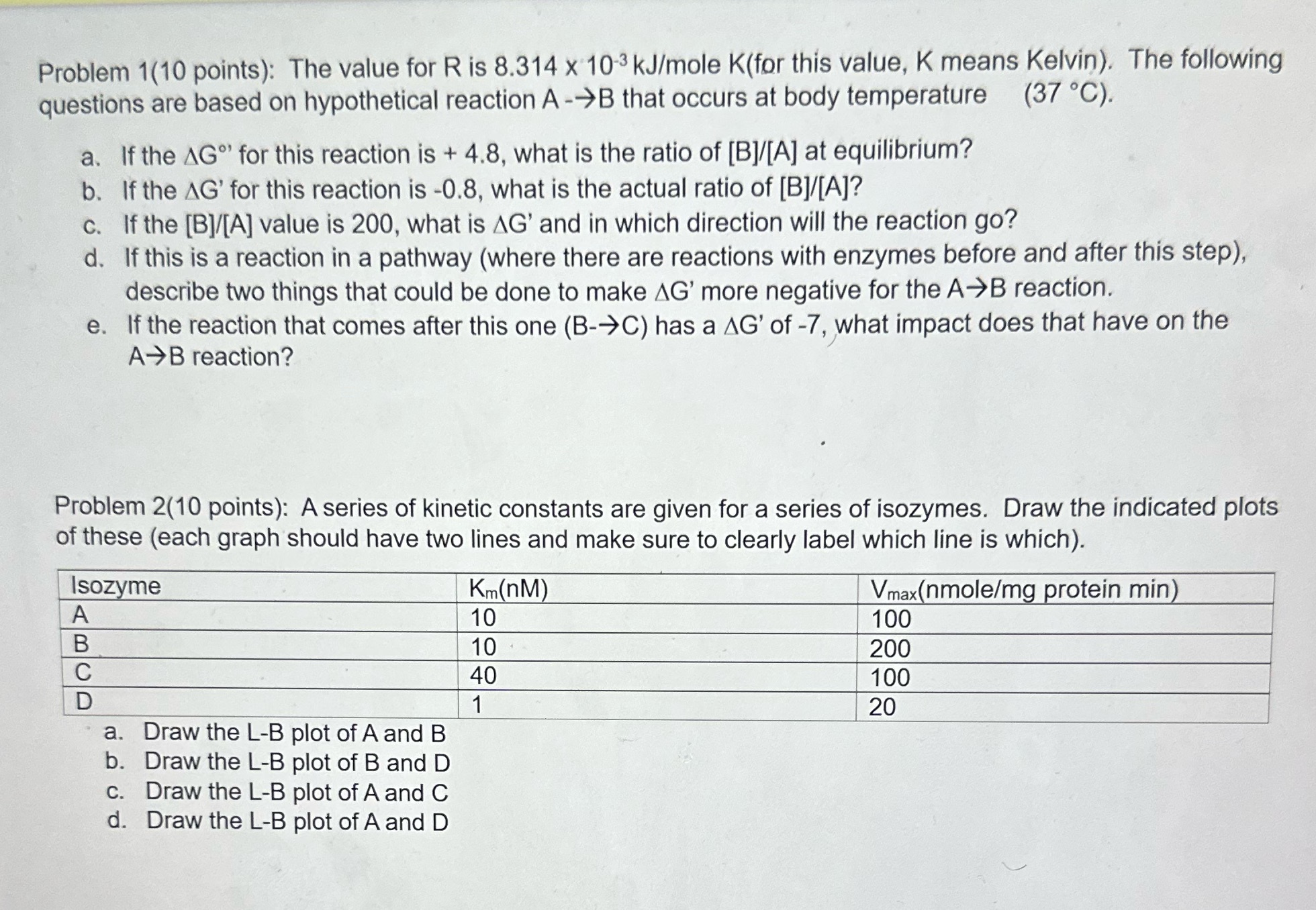
Problem 1(10 points): The value for R is 8.314 x 10'3 kJ/mole K(fpr this value, K means Kelvin). The following questions are based on hypothetical reaction A --)B that occurs at body temperature (37 C). a. if the AG\" for this reaction is + 4.8, what is the ratio of [Bl/[A] at equilibrium? b. If the AG' for this reaction is -0.8, what is the actual ratio of [B]/[A]? c. if the [Bl/[A] value is 200, what is AG' and in which direction will the reaction go? d. If this is a reaction in a pathway (where there are reactions with enzymes before and after this step), describe two things that could be done to make AG' more negative for the A98 reaction. e. if the reaction that comes after this one (B-C) has a AG' of -7, [what impact does that have on the A98 reaction? / Problem 2(10 points): A series of kinetic constants are given for a series of isozymes. Draw the indicated plots of these (each graph should have two lines and make sure to clearly label which line is which). Vmax nmole/m rotein min i A 10 i B 10 i C 40 D 1 20 i a. Draw the LB plot oannd B b. Draw the L-B plot of B and D c. Draw the LB plot of A and C (1. Draw the L-B plot of A and D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


