Question: Problem 13.76 - Enhanced - with Feedback Using data from the table, calculate the troozing and boiling points of each of the following solutions Part
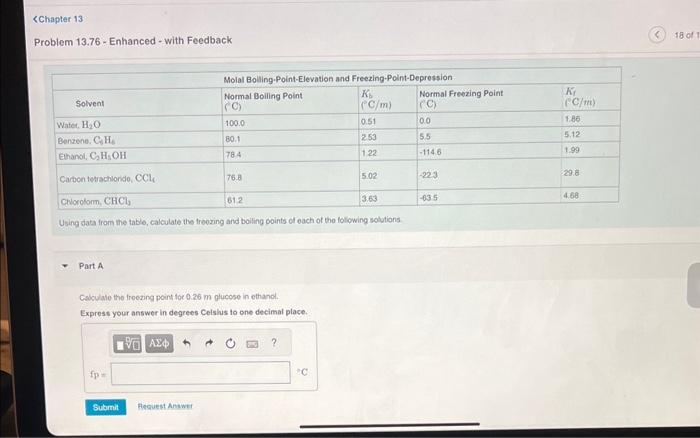
Problem 13.76 - Enhanced - with Feedback Using data from the table, calculate the troozing and boiling points of each of the following solutions Part A Cabculale the froezing point foc 0.26m plucose in ethand. Exprest your answer in degrees Celshus to one decimal place. Problem 13.76 - Enhanced - with Feedback Part B Calculate the boiling point for 0.26m glucose in ethanol. Express your answer in degrees Celsius to one decimal place. Part C Calculate the freezing point for 22.0g of decane, C10H22, in 46.5gCHCl3. Express your answer in degrees Celsius to one decimal place. Calculate the boiling point for 22.0g of decane, C10H22, in 46.5gCHCl. Express your answer in degrees Celsius to one decimal place. Part E Calculate the freezing point for 4.00gNaOH in 185g of water. Express your answer in degrees Celsius to one decimal place. Calculate the boiling point for 4.00gNaOH in 185g of water. Express your answer in degrees Celsius to one decimal place. Part G Calculate the freezing point for 0.48mol ethylene glycol and 0.18molKBr in 148gH2O. Express your answer in degrees Celsius to one decimal place. Calculate the boiling point for 0.48mol ethylene glycol and 0.18molKBr in 148gH2O. Express your answer in degrees Celsius to one decimal place
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


