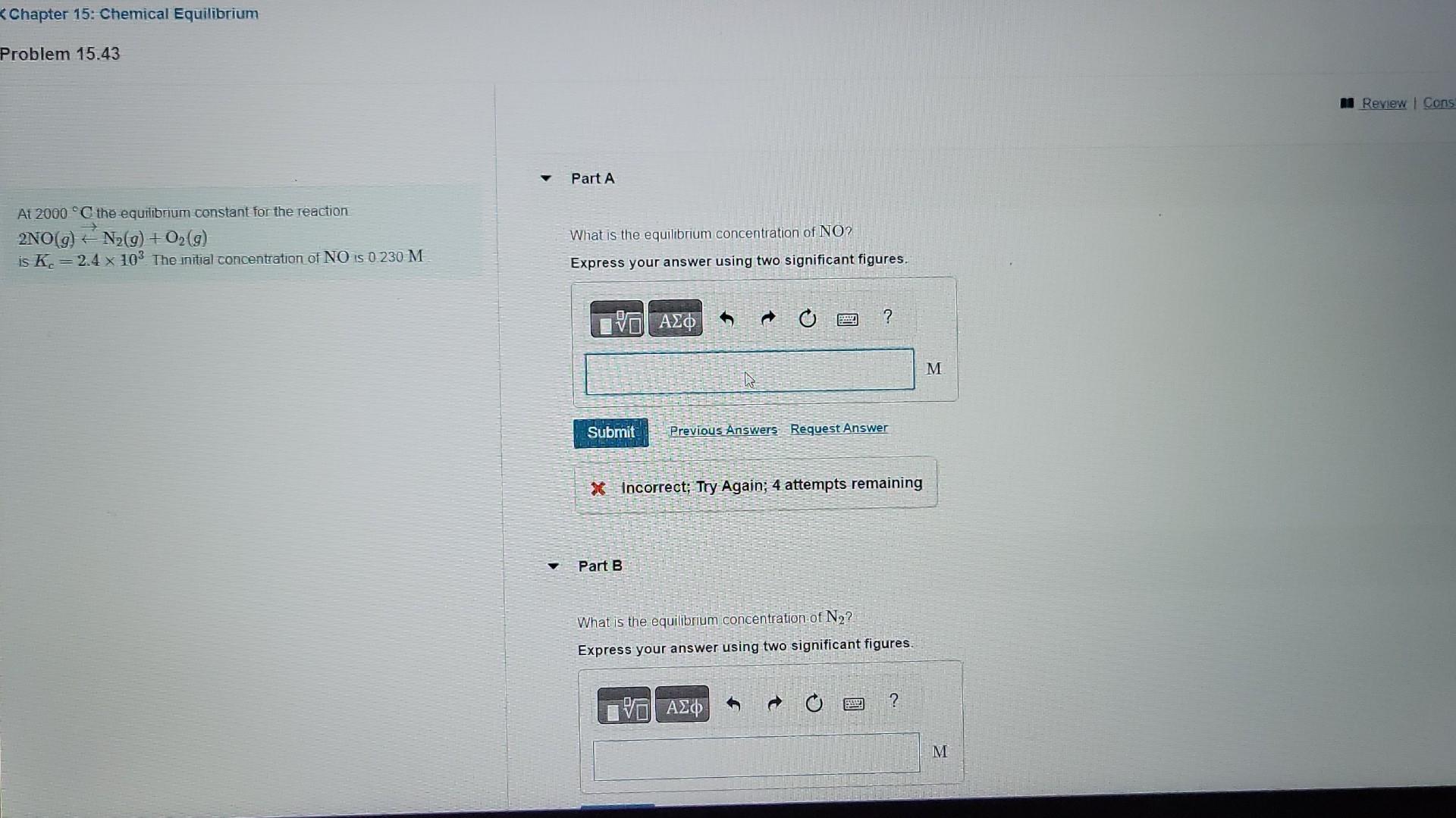
Question: problem 15.43 Chapter 15: Chemical Equilibrium Problem 15.43 Part A At 2000C the equilibrium constant for the reaction. 2NO(g)N2(g)+O2(g) What is the equilibrium concentration of
problem 15.43
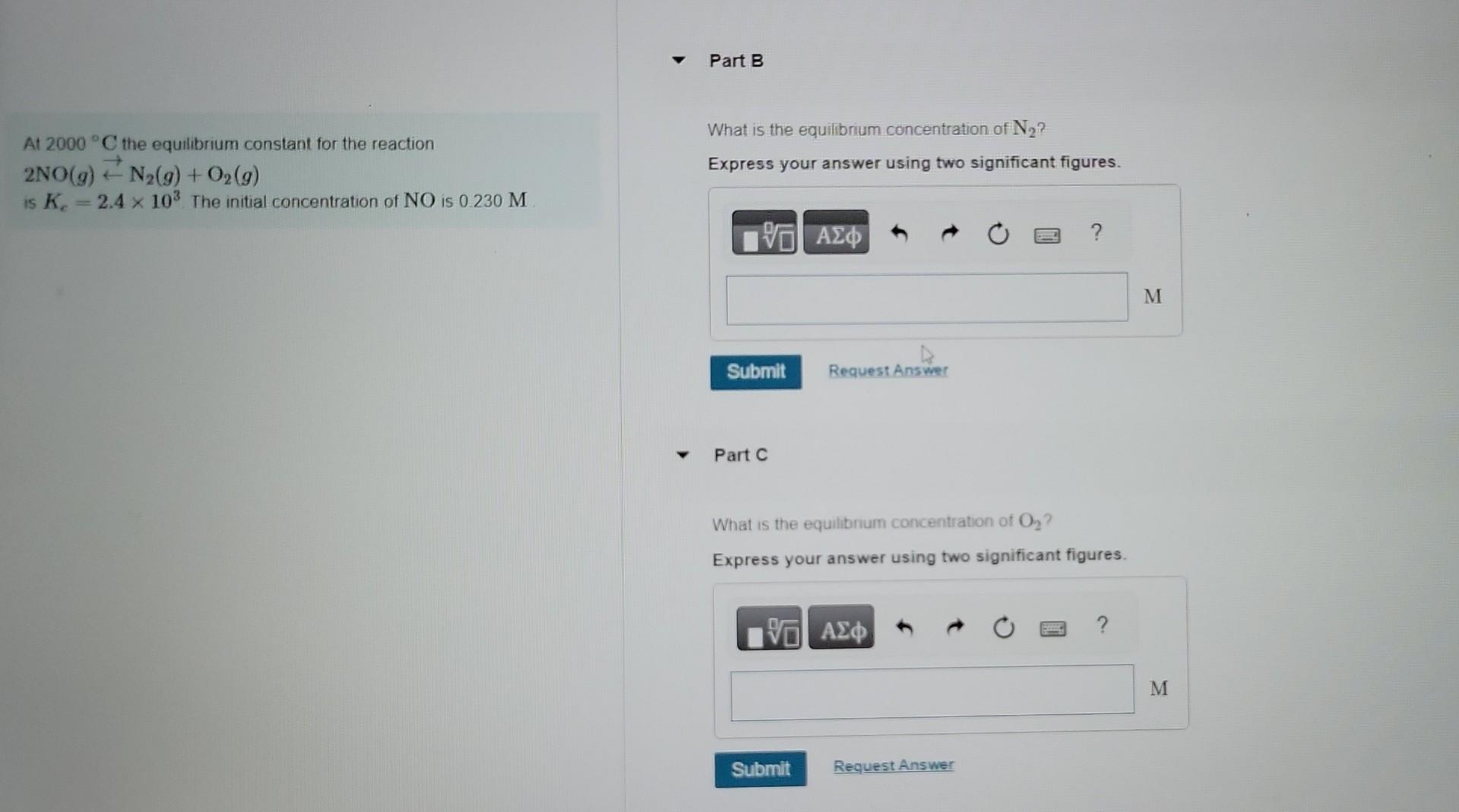
Chapter 15: Chemical Equilibrium Problem 15.43 Part A At 2000C the equilibrium constant for the reaction. 2NO(g)N2(g)+O2(g) What is the equilibrium concentration of NO? is Kc=2.4103. The initial concentration of NO is 0.230M Express your answer using two significant figures. if Incorrect; Try Again; 4 attempts remaining Part B What is the equilibrium concentration of N2 ? Express your answer using two significant figures. At 2000C the equilibrium constant for the reaction What is the equilibrium concentration of N2 ? 2NO(g)N2(g)+O2(g) Express your answer using two significant figures. is Kc=2.4103. The initial concentration of NO is 0.230M Part C What is the equilibnum concentration of O2 ? Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


