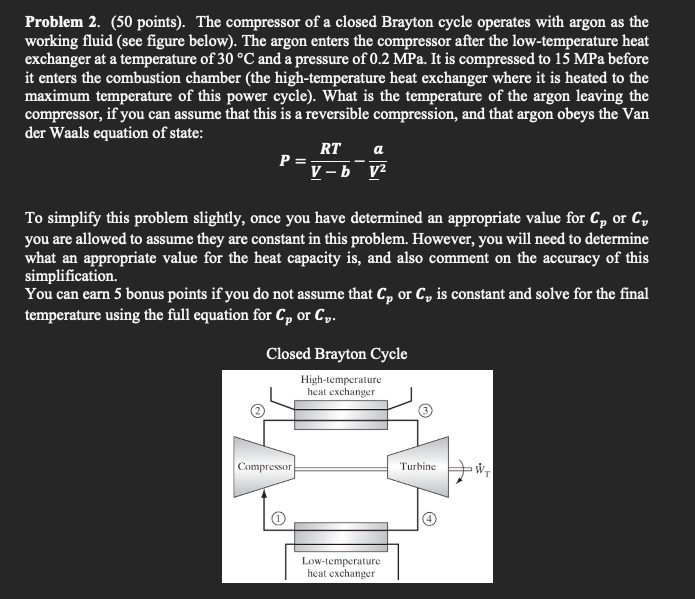
Question: Problem 2 . ( 5 0 points ) . The compressor of a closed Brayton cycle operates with argon as the working fluid ( see
Problem points The compressor of a closed Brayton cycle operates with argon as the working fluid see figure below The argon enters the compressor after the lowtemperature heat exchanger at a temperature of circmathrmC and a pressure of MPa It is compressed to MPa before it enters the combustion chamber the hightemperature heat exchanger where it is heated to the maximum temperature of this power cycle What is the temperature of the argon leaving the compressor, if you can assume that this is a reversible compression, and that argon obeys the Van der Waals equation of state:
PfracR TunderlineVbfracaunderlineV
To simplify this problem slightly, once you have determined an appropriate value for boldsymbolCboldsymbolp or boldsymbolCboldsymbolv you are allowed to assume they are constant in this problem. However, you will need to determine what an appropriate value for the heat capacity is and also comment on the accuracy of this simplification.
You can earn bonus points if you do not assume that Cp or Cv is constant and solve for the final temperature using the full equation for Cp or Cv
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


