Question: Problem 2 (50 points): The first order, reversible reaction AB+ 2C is taking place in a membrane reactor. Pure A enters the reactor, and B
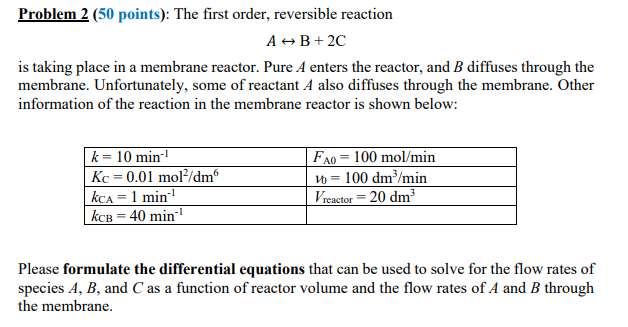
Problem 2 (50 points): The first order, reversible reaction AB+ 2C is taking place in a membrane reactor. Pure A enters the reactor, and B diffuses through the membrane. Unfortunately, some of reactant A also diffuses through the membrane. Other information of the reaction in the membrane reactor is shown below: k= 10 min-1 Kc = 0.01 mol/dm kca = 1 min kce = 40 min-1 FA0 = 100 mol/min 10 = 100 dm/min Vreactor = 20 dm? Please formulate the differential equations that can be used to solve for the flow rates of species A, B, and C as a function of reactor volume and the flow rates of A and B through the membrane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


