Question: Problem 2. A fractionation column operating at 101 kPa is to separate 3000 kg/hr of a mixture of benzene and toluene containing 0.3 mass fraction
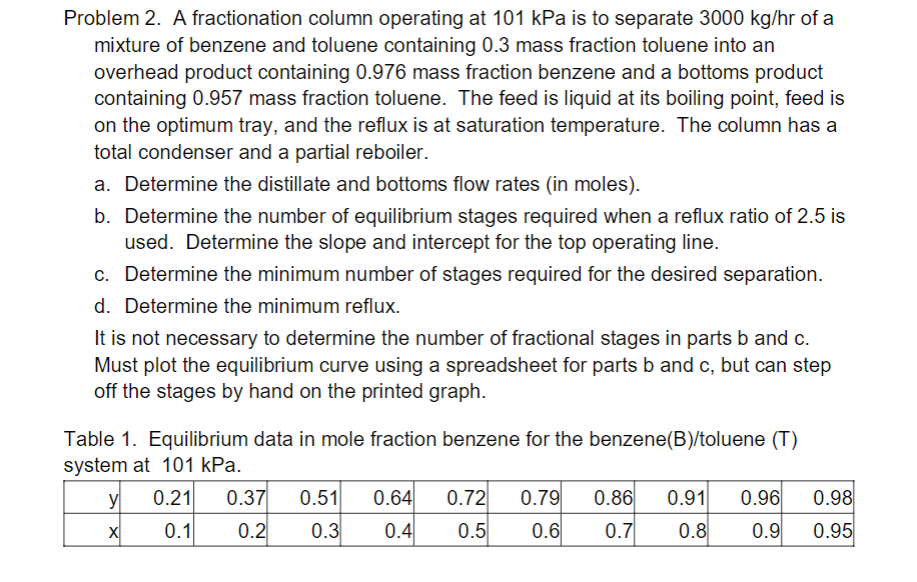
Problem 2. A fractionation column operating at 101 kPa is to separate 3000 kg/hr of a mixture of benzene and toluene containing 0.3 mass fraction toluene into an overhead product containing 0.976 mass fraction benzene and a bottoms product containing 0.957 mass fraction toluene. The feed is liquid at its boiling point, feed is on the optimum tray, and the reflux is at saturation temperature. The column has a total condenser and a partial reboiler. a. Determine the distillate and bottoms flow rates (in moles). b. Determine the number of equilibrium stages required when a reflux ratio of 2.5 is used. Determine the slope and intercept for the top operating line. c. Determine the minimum number of stages required for the desired separation. d. Determine the minimum reflux. It is not necessary to determine the number of fractional stages in parts b and c. Must plot the equilibrium curve using a spreadsheet for parts b and c, but can step off the stages by hand on the printed graph. Table 1. Equilibrium data in mole fraction benzene for the benzene(B)/toluene (T) system at 101 kPa. yi 0.21 0.37 0.51 0.64 0.72 0.79 0.86 0.91 0.96 X 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 0.98 0.95
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


