Question: Problem 2: Diffusion in the Atmosphere (a) A point source of 100 g of SO2 is released. If molecular diffusion (D = 0.12 cm's 1)
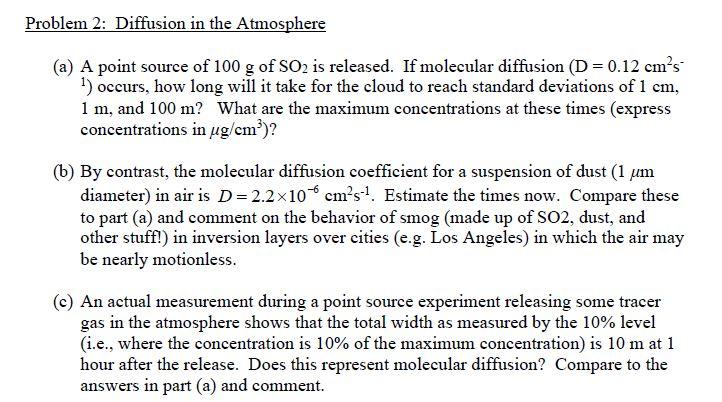
Problem 2: Diffusion in the Atmosphere (a) A point source of 100 g of SO2 is released. If molecular diffusion (D = 0.12 cm's 1) occurs, how long will it take for the cloud to reach standard deviations of 1 cm, 1 m, and 100 m? What are the maximum concentrations at these times (express concentrations in ug/cm)? (b) By contrast, the molecular diffusion coefficient for a suspension of dust (1 um diameter) in air is D=2.2x10 cms-1. Estimate the times now. Compare these to part (a) and comment on the behavior of smog (made up of S02, dust, and other stuff!) in inversion layers over cities (e.g. Los Angeles) in which the air may be nearly motionless. (c) An actual measurement during a point source experiment releasing some tracer gas in the atmosphere shows that the total width as measured by the 10% level (i.e., where the concentration is 10% of the maximum concentration) is 10 m at 1 hour after the release. Does this represent molecular diffusion? Compare to the answers in part (a) and comment. Problem 2: Diffusion in the Atmosphere (a) A point source of 100 g of SO2 is released. If molecular diffusion (D = 0.12 cm's 1) occurs, how long will it take for the cloud to reach standard deviations of 1 cm, 1 m, and 100 m? What are the maximum concentrations at these times (express concentrations in ug/cm)? (b) By contrast, the molecular diffusion coefficient for a suspension of dust (1 um diameter) in air is D=2.2x10 cms-1. Estimate the times now. Compare these to part (a) and comment on the behavior of smog (made up of S02, dust, and other stuff!) in inversion layers over cities (e.g. Los Angeles) in which the air may be nearly motionless. (c) An actual measurement during a point source experiment releasing some tracer gas in the atmosphere shows that the total width as measured by the 10% level (i.e., where the concentration is 10% of the maximum concentration) is 10 m at 1 hour after the release. Does this represent molecular diffusion? Compare to the answers in part (a) and comment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


