Question: Problem 2 Figure ( i ) shows a hard - sphere model for a hexagonal close - packed ( HCP ) unit cell, whereas Figures
Problem
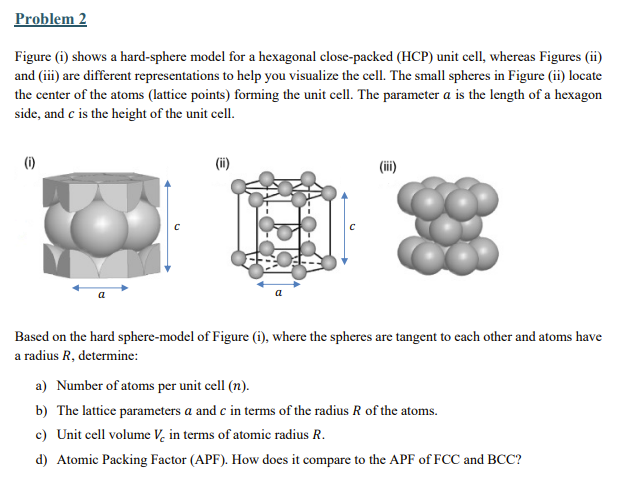
Figure i shows a hardsphere model for a hexagonal closepacked HCP unit cell, whereas Figures ii
and iii are different representations to help you visualize the cell. The small spheres in Figure ii locate
the center of the atoms lattice points forming the unit cell. The parameter is the length of a hexagon
side, and is the height of the unit cell.
Based on the hard spheremodel of Figure i where the spheres are tangent to each other and atoms have
a radius determine:
a Number of atoms per unit cell
b The lattice parameters a and in terms of the radius of the atoms.
c Unit cell volume in terms of atomic radius
d Atomic Packing Factor APF How does it compare to the APF of FCC and BCC
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


