Question: Problem 2: Find the equilibrium temperature attained by a porous surface soaked in toluene. The surrounding air, at pressure p = 101.325 kPa and temperature
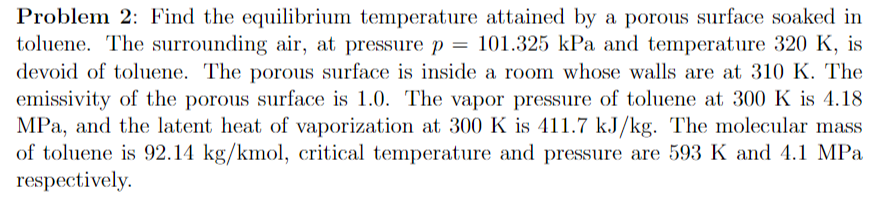
Problem 2: Find the equilibrium temperature attained by a porous surface soaked in toluene. The surrounding air, at pressure p = 101.325 kPa and temperature 320 K, is devoid of toluene. The porous surface is inside a room whose walls are at 310 K. The emissivity of the porous surface is 1.0. The vapor pressure of toluene at 300 K is 4.18 MPa, and the latent heat of vaporization at 300 K is 411.7 kJ/kg. The molecular mass of toluene is 92.14 kg/kmol, critical temperature and pressure are 593 K and 4.1 MPa respectively
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


