Question: Problem 2 . For the water gas shift reaction, evaluate the equilibrium constants at between 2 9 8 K and 1 0 0 0 K
Problem For the water gas shift reaction, evaluate the equilibrium constants at
between and and at an atmospheric pressure and the corresponding
equilibrium fractional conversion values of using the thermodynamic data
reported in the literature. In these calculations, consider a feed stream containing
and inerts.
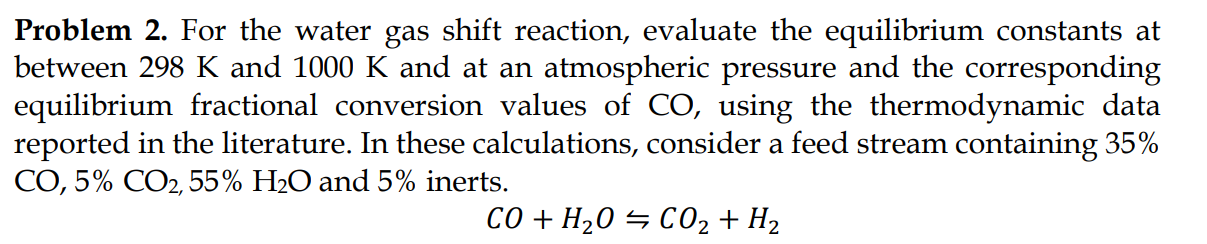
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


