Question: Problem # 2 : NaOH ( a q ) is often used to titente H C l ( a q ) . H C l
Problem #: NaOH is often used to titente
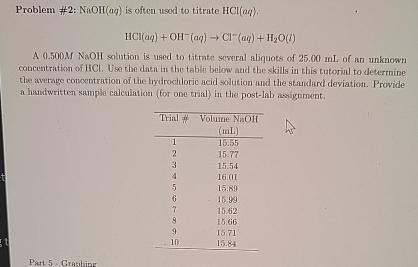
A MNaOH solution is used to titrate several aliquats of of an unknown concentration of Use the datn in the tablin below and the skils in this tutorial to determine the aserage concentration of the hydrochloric acid solution and the standard deviation. Prowide a handwritter sample calctiation for one trial in the postlab asignment.
tableTrial ##tableVolume NaOH
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


