Question: Problem 2 Solve part B ! ! ! ! 2 of 8 Review Constants Part A High - altitude mountain climbers do not eat snow,
Problem Solve part B
of
Review
Constants
Part A
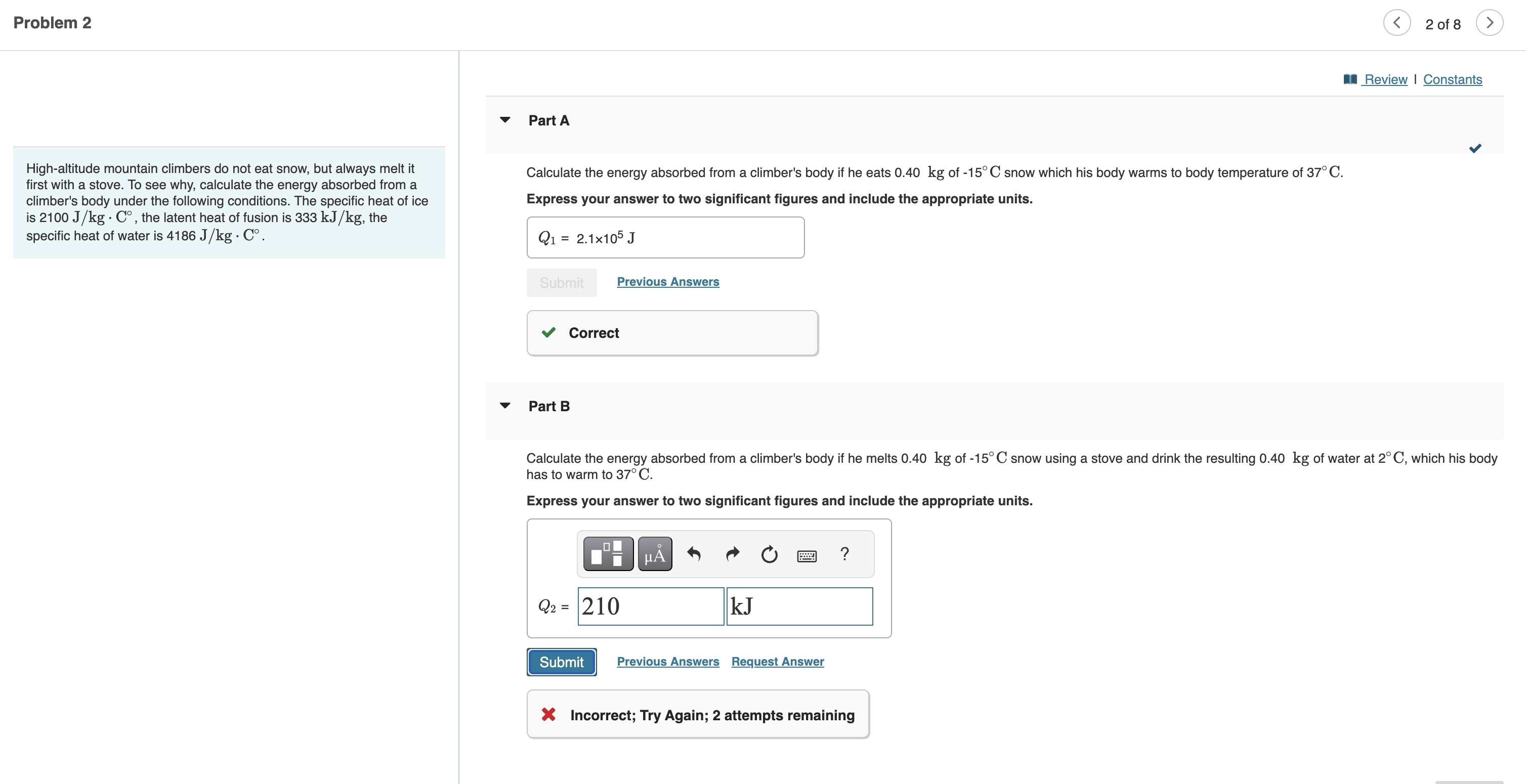
Highaltitude mountain climbers do not eat snow, but always melt it first with a stove. To see why, calculate the energy absorbed from a climber's body under the following conditions. The specific heat of ice is the latent heat of fusion is the specific heat of water is
Calculate the energy absorbed from a climber's body if he eats kg of snow which his body warms to body temperature of
Express your answer to two significant figures and include the appropriate units.
Previous Answers
Correct
Part B
Calculate the energy absorbed from a climber's body if he melts kg of snow using a stove and drink the resulting kg of water at which his body has to warm to
Express your answer to two significant figures and include the appropriate units.
Previous Answers
Request Answer
Incorrect; Try Again; attempts remainin
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


