Question: Problem 2. The famous second explosion limit in the H2O2 reaction system is dictated by the competition between the growth of the H atom via
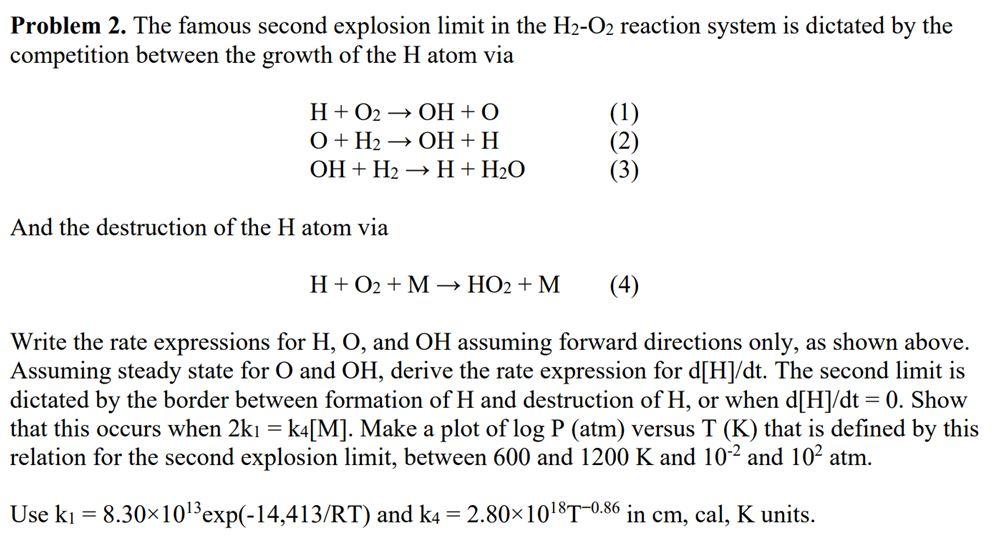
Problem 2. The famous second explosion limit in the H2O2 reaction system is dictated by the competition between the growth of the H atom via H+O2OH+OO+H2OH+HOH+H2H+H2O And the destruction of the H atom via H+O2+MHO2+M Write the rate expressions for H,O, and OH assuming forward directions only, as shown above. Assuming steady state for O and OH, derive the rate expression for d[H]/dt. The second limit is dictated by the border between formation of H and destruction of H, or when d[H]/dt=0. Show that this occurs when 2k1=k4[M]. Make a plot of logP(atm) versus T(K) that is defined by this relation for the second explosion limit, between 600 and 1200K and 102 and 102 atm. Use k1=8.301013exp(14,413/RT) and k4=2.801018T0.86 in cm, cal, K units. Problem 2. The famous second explosion limit in the H2O2 reaction system is dictated by the competition between the growth of the H atom via H+O2OH+OO+H2OH+HOH+H2H+H2O And the destruction of the H atom via H+O2+MHO2+M Write the rate expressions for H,O, and OH assuming forward directions only, as shown above. Assuming steady state for O and OH, derive the rate expression for d[H]/dt. The second limit is dictated by the border between formation of H and destruction of H, or when d[H]/dt=0. Show that this occurs when 2k1=k4[M]. Make a plot of logP(atm) versus T(K) that is defined by this relation for the second explosion limit, between 600 and 1200K and 102 and 102 atm. Use k1=8.301013exp(14,413/RT) and k4=2.801018T0.86 in cm, cal, K units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


