Question: Problem 2 The molar enthalpy of a binary liquid mixture of species 1 and 2 is given by: H = a x 1 + b
Problem
The molar enthalpy of a binary liquid mixture of species and is given by:
where
where the temperature is in Kelvin.
a Derive the expression for the partial molar enthalpy of the species
b Using the result of a calculate the infinite dilution value for the partial molar enthalpy of the species at the temperature
c Calculate the enthalpy of mixing in J of a mixture with moles of species I and moles of species at
d Consider the adiabatic mixing of a stream of pure flowing at with a stream of pure flowing at molls. Both
inlet streams are at C What is the exit temperature of the mixture?
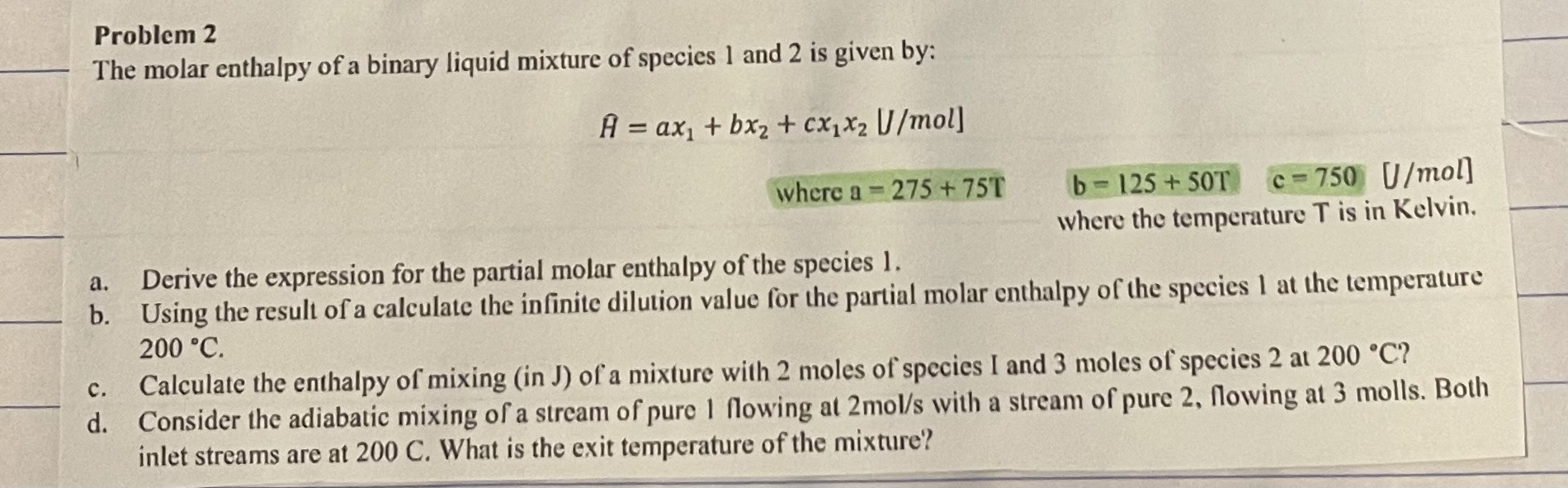
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


