Question: Problem 2: Two rivers flow into a dammed lake in a tranquil watershed in northern New Mexico, one (River A) originating from snow-covered high mountain
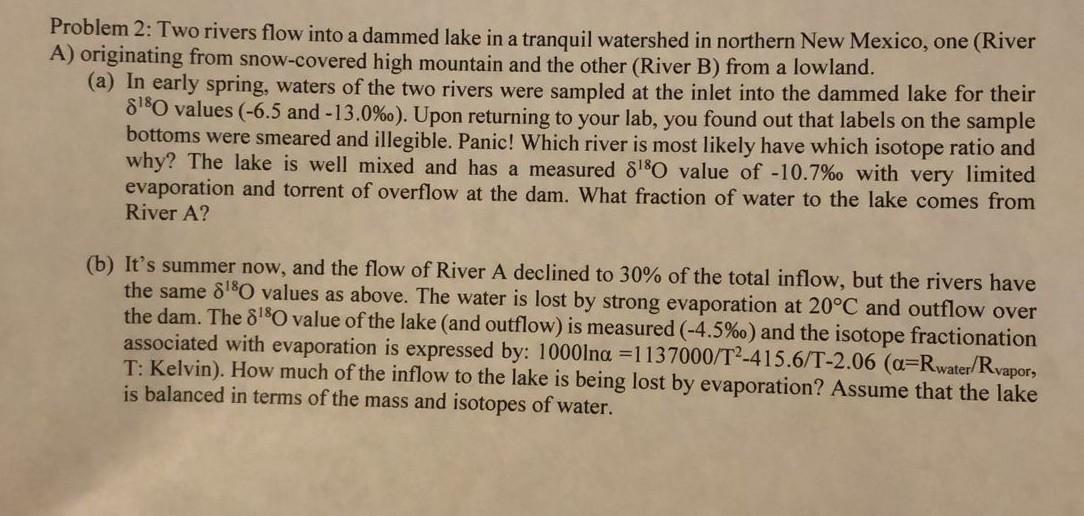
Problem 2: Two rivers flow into a dammed lake in a tranquil watershed in northern New Mexico, one (River A) originating from snow-covered high mountain and the other (River B) from a lowland. (a) In early spring, waters of the two rivers were sampled at the inlet into the dammed lake for their 8180 values (-6.5 and -13.0%o). Upon returning to your lab, you found out that labels on the sample bottoms were smeared and illegible. Panic! Which river is most likely have which isotope ratio and why? The lake is well mixed and has a measured 8180 value of -10.7%o with very limited evaporation and torrent of overflow at the dam. What fraction of water to the lake comes from River A? (b) It's summer now, and the flow of River A declined to 30% of the total inflow, but the rivers have the same 8'80 values as above. The water is lost by strong evaporation at 20C and outflow over the dam. The 8180 value of the lake (and outflow) is measured (-4.5%o) and the isotope fractionation associated with evaporation is expressed by: 1000lna =1137000/T2-415.6/T-2.06 (a=Rwater/Rvapor, T: Kelvin). How much of the inflow to the lake is being lost by evaporation? Assume that the lake is balanced in terms of the mass and isotopes of water. Problem 2: Two rivers flow into a dammed lake in a tranquil watershed in northern New Mexico, one (River A) originating from snow-covered high mountain and the other (River B) from a lowland. (a) In early spring, waters of the two rivers were sampled at the inlet into the dammed lake for their 8180 values (-6.5 and -13.0%o). Upon returning to your lab, you found out that labels on the sample bottoms were smeared and illegible. Panic! Which river is most likely have which isotope ratio and why? The lake is well mixed and has a measured 8180 value of -10.7%o with very limited evaporation and torrent of overflow at the dam. What fraction of water to the lake comes from River A? (b) It's summer now, and the flow of River A declined to 30% of the total inflow, but the rivers have the same 8'80 values as above. The water is lost by strong evaporation at 20C and outflow over the dam. The 8180 value of the lake (and outflow) is measured (-4.5%o) and the isotope fractionation associated with evaporation is expressed by: 1000lna =1137000/T2-415.6/T-2.06 (a=Rwater/Rvapor, T: Kelvin). How much of the inflow to the lake is being lost by evaporation? Assume that the lake is balanced in terms of the mass and isotopes of water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


