Question: Problem 26-2E. Consider the simplified process shown in Figure 26-2 below, which is designed to treat a mixture gas phase mixture of TCE in air
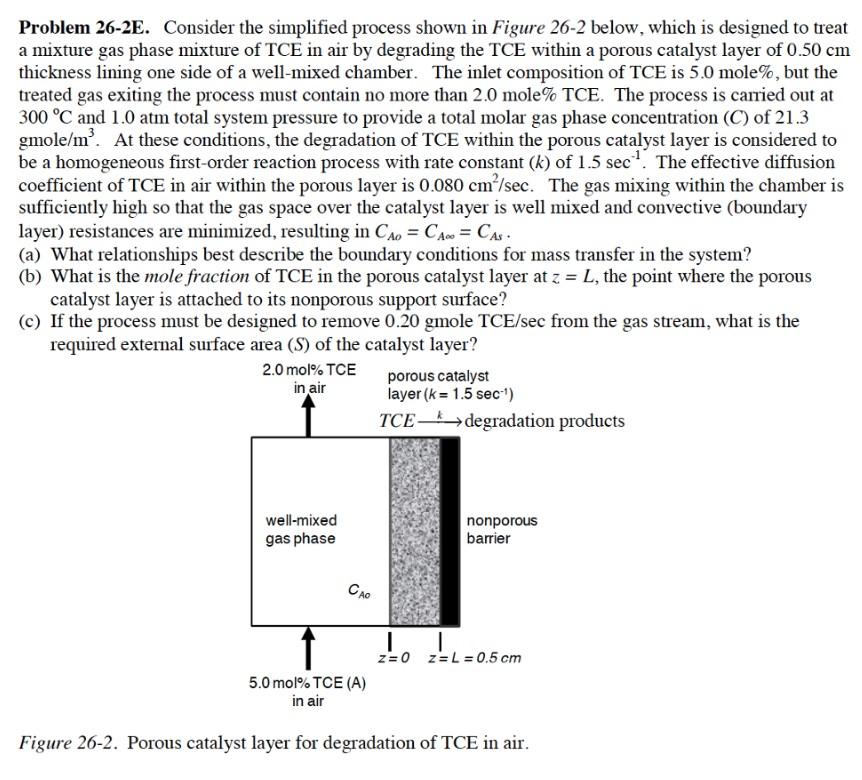
Problem 26-2E. Consider the simplified process shown in Figure 26-2 below, which is designed to treat a mixture gas phase mixture of TCE in air by degrading the TCE within a porous catalyst layer of 0.50 cm thickness lining one side of a well-mixed chamber. The inlet composition of TCE is 5.0 mole%, but the treated gas exiting the process must contain no more than 2.0 mole% TCE. The process is carried out at 300 C and 1.0 atm total system pressure to provide a total molar gas phase concentration (C) of 21.3 gmole/m'. At these conditions, the degradation of TCE within the porous catalyst layer is considered to be a homogeneous first-order reaction process with rate constant (k) of 1.5 sec? The effective diffusion coefficient of TCE in air within the porous layer is 0.080 cm /sec. The gas mixing within the chamber is sufficiently high so that the gas space over the catalyst layer is well mixed and convective (boundary layer) resistances are minimized, resulting in CA = CA = CAs. (a) What relationships best describe the boundary conditions for mass transfer in the system? (b) What is the mole fraction of TCE in the porous catalyst layer at z = L, the point where the porous catalyst layer is attached to its nonporous support surface? c) If the process must be designed to remove 0.20 gmole TCE/sec from the gas stream, what is the required external surface area (S) of the catalyst layer? 2.0 mol% TCE porous catalyst in air layer (k = 1.5 sec-1) TCE-* degradation products well-mixed gas phase nonporous barrier CAO 1 | FL=0.50 z=0 z= L = 0.5 cm 5.0 mol% TCE (A) in air Figure 26-2. Porous catalyst layer for degradation of TCE in air. Problem 26-2E. Consider the simplified process shown in Figure 26-2 below, which is designed to treat a mixture gas phase mixture of TCE in air by degrading the TCE within a porous catalyst layer of 0.50 cm thickness lining one side of a well-mixed chamber. The inlet composition of TCE is 5.0 mole%, but the treated gas exiting the process must contain no more than 2.0 mole% TCE. The process is carried out at 300 C and 1.0 atm total system pressure to provide a total molar gas phase concentration (C) of 21.3 gmole/m'. At these conditions, the degradation of TCE within the porous catalyst layer is considered to be a homogeneous first-order reaction process with rate constant (k) of 1.5 sec? The effective diffusion coefficient of TCE in air within the porous layer is 0.080 cm /sec. The gas mixing within the chamber is sufficiently high so that the gas space over the catalyst layer is well mixed and convective (boundary layer) resistances are minimized, resulting in CA = CA = CAs. (a) What relationships best describe the boundary conditions for mass transfer in the system? (b) What is the mole fraction of TCE in the porous catalyst layer at z = L, the point where the porous catalyst layer is attached to its nonporous support surface? c) If the process must be designed to remove 0.20 gmole TCE/sec from the gas stream, what is the required external surface area (S) of the catalyst layer? 2.0 mol% TCE porous catalyst in air layer (k = 1.5 sec-1) TCE-* degradation products well-mixed gas phase nonporous barrier CAO 1 | FL=0.50 z=0 z= L = 0.5 cm 5.0 mol% TCE (A) in air Figure 26-2. Porous catalyst layer for degradation of TCE in air
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


