Question: Problem 3. A distillation column is separating benzene (B) from chlorobenzene (C) at atmospheric pressure. The column has two feed streams. The first feed is
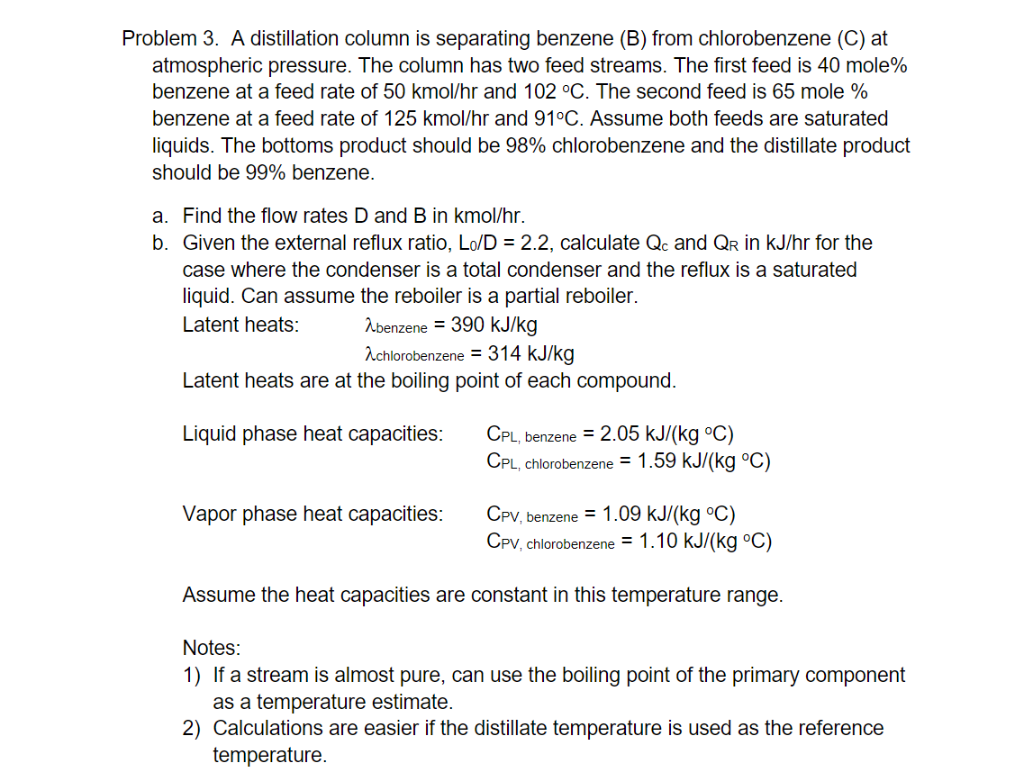
Problem 3. A distillation column is separating benzene (B) from chlorobenzene (C) at atmospheric pressure. The column has two feed streams. The first feed is 40 mole% benzene at a feed rate of 50 kmol/hr and 102 C. The second feed is 65 mole % benzene at a feed rate of 125 kmol/hr and 91C. Assume both feeds are saturated liquids. The bottoms product should be 98% chlorobenzene and the distillate product should be 99% benzene. a. Find the flow rates D and B in kmol/hr. b. Given the external reflux ratio, Lo/D = 2.2, calculate Qc and QR in kJ/hr for the case where the condenser is a total condenser and the reflux is a saturated liquid. Can assume the reboiler is a partial reboiler. Latent heats: abenzene = 390 kJ/kg achlorobenzene = 314 kJ/kg Latent heats are at the boiling point of each compound. Liquid phase heat capacities: CPL, benzene = 2.05 kJ/(kg C) CPL, chlorobenzene = 1.59 kJ/(kg C) Vapor phase heat capacities: Cpv, benzene = 1.09 kJ/(kg C) Cpv, chlorobenzene = 1.10 kJ/(kg C) Assume the heat capacities are constant in this temperature range. Notes: 1) If a stream is almost pure, can use the boiling point of the primary component as a temperature estimate. 2) Calculations are easier if the distillate temperature is used as the reference temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


